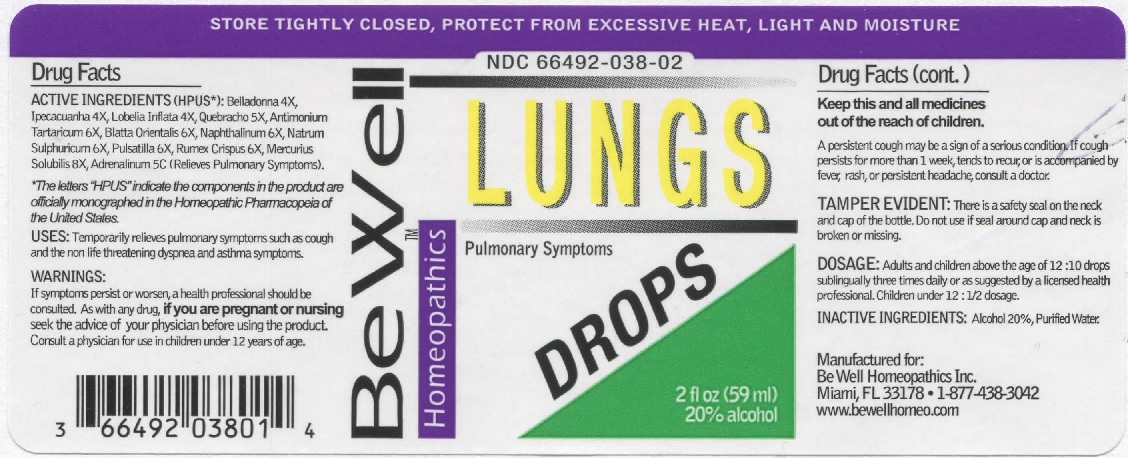
LUNG DROPS- belladonna, ipecacuanha, lobelia inflata, quebracho, antimonium tartaricum, blatta orientalis, naphthalinum, natrum sulphuricum, pulsatilla, rumex crispus, mercurius solubilis, adrenalinum. liquid
Be Well Medical dba Richard Clement Nutrition y Be Well Homeopathics
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Lung Drops
ACTIVE INGREDIENTS (HPUS*): Belladonna 4X, Ipecacuanha 4X, Lobelia Inflata 4X, Quebracho 5X, Antimonium Tartaricum 6X, Blatta Orientalis 6X, Naphthalinum 6X, Natrum Sulphuricum 6X, Pulsatilla 6X, Rumex Crispus 6X, Mercurius Solubilis 8X, Adrenalinum 5C (Relieves Pulmonary Symptoms).
*The letters "HPUS" indicate that the components in the product are officially monographed in the Homeopathic Pharmacopeia of the United States.
USES: Temporarily relieves pulmonary symptoms such as cough and the non life threatening dyspnea and asthma symptoms.
WARNINGS: If symptoms persist or worsen, a health professional should be consulted. As with any drug, if you are pregnant or nursing seek the advice of your physician before using this product. Consult a physician for use in children under 12 years of age.
DOSAGE: Adults and children above the age of 12: 10 drops sublingually three times daily or as suggested by a licensed health professional. Children under 12: 1/2 dosage.
| LUNG DROPS
belladonna, ipecacuanha, lobelia inflata, quebracho, antimonium tartaricum, blatta orientalis, naphthalinum, natrum sulphuricum, pulsatilla, rumex crispus, mercurius solubilis, adrenalinum. liquid |
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
| Labeler - Be Well Medical dba Richard Clement Nutrition y Be Well Homeopathics (052584997) |