URAMAXIN TS- urea cream
Medimetriks Pharmaceuticals, inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Uramaxin(45% Urea)TS
PATIENT INSTRUCTIONS
1. Apply Uramaxin TS to affected skin twice per day, or as directed by a physician.
2. For best results, apply Uramaxin TS to moistened skin, such as after a shower or bath.
3. After Uramaxin TS has dried completely, if any excess appears, simply wipe skin with a damp cloth.
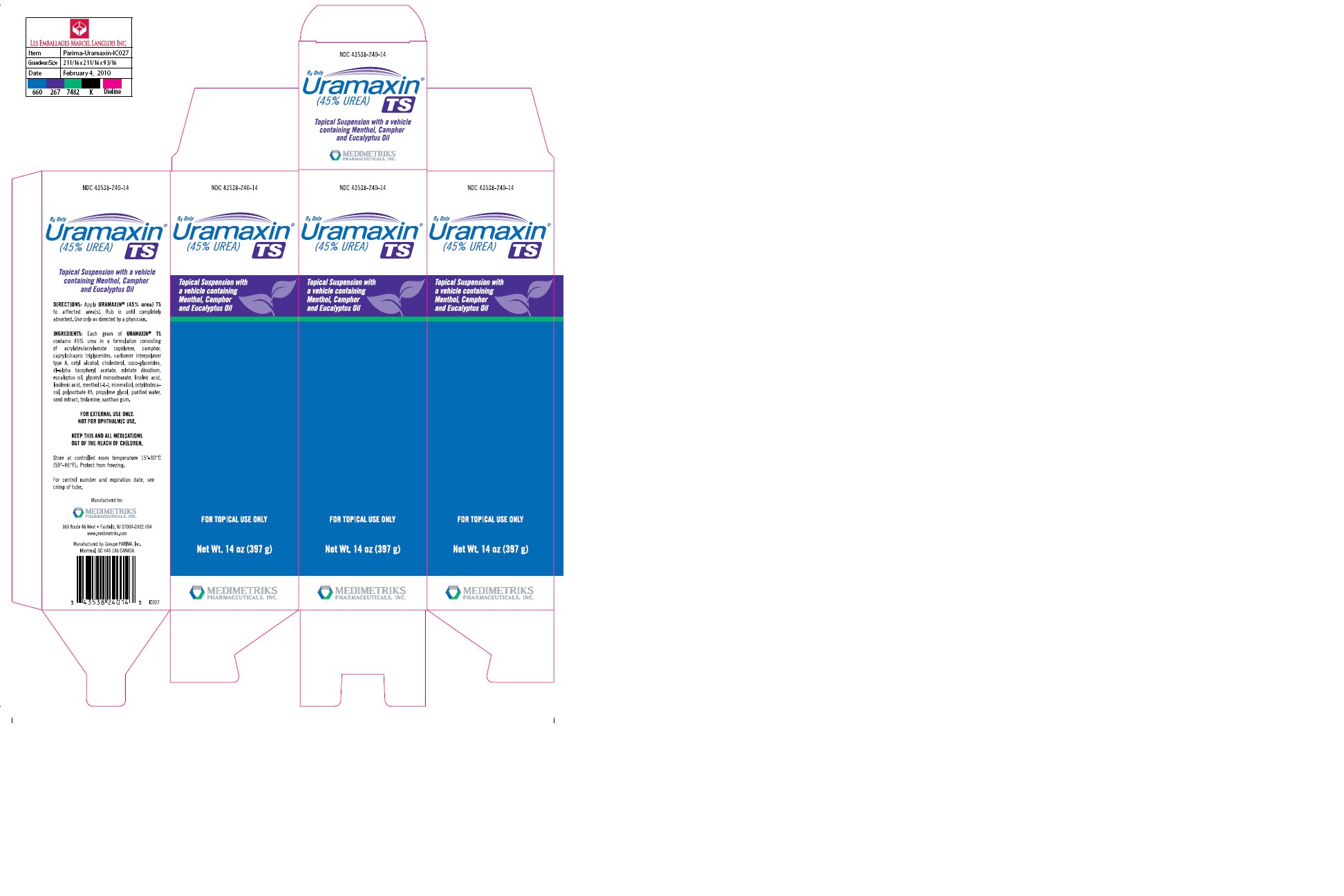
DESCRIPTION: Uramaxin (45% Urea) TS is a keratolytic emollient, which is a gentle, yet potent, tissue softener for nails and/or skin. Each gram of Uramaxin (45% Urea) TS contains 45% urea in a formulation consisting of acrylates/acrylamide copolymer, camphor, caprylic/capric triglycerides, carbomer interpolymer type A, cetyl alcohol, cholesterol, coco-glycerides, dl-alpha tocopheryl acetate, edetate disodium, eucalyptus oil, glyceryl monostearate, linoleic acid, linolenic acid, menthol (-L-), mineral oil, octyldodecanol, polysorbate 85, propylene glycol, purified water, seed extract, trolamine, xanthan gum.
Urea is a diamide of carbonic acid with the following chemical structure:

CLINICAL PHARMACOLOGY: Urea gently dissolves the intercellular matrix, which results in loosening the horny layer of skin and shedding scaly skin at regular intervals, thereby softening hyperkeratotic areas.
INDICATION AND USES: For debridement and promotion of normal healing of hyperkeratotic surface lesions, particularly where healing is retarded by local infection, necrotic tissue, fibrinous or purulent debris or eschar. Urea is useful for the treatment of hyperkeratotic conditions such as dry, rough skin, dermatitis, psoriasis, xerosis, ichthyosis, eczema, keratosis, keratoderma, corns and calluses.
PRECAUTIONS: This medication is to be used as directed by a physician and should not be used to treat any condition other than that for which it was prescribed. If redness or irritation occurs, discontinue use.
PREGNANCY: Pregnancy Category B. Animal reproduction studies have revealed no evidence of harm to the fetus, however, there are no adequate and well-controlled studies in pregnant women. Because animal reproductive studies are not always predictive of human response, Uramaxin (45% Urea) TS should be given to pregnant women only if clearly needed.
NURSING MOTHERS: It is not known whether or not this drug is secreted in human milk. Because many drugs are secreted in human milk, caution should be exercised when Uramaxin (45% Urea) TS is administered to a nursing woman.
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
ADVERSE REACTIONS: Transient stinging, burning, itching or irritation may occur and normally disappear on discontinuing the medication.
DOSAGE AND ADMINISTRATION: Apply Uramaxin (45% Urea) TS to affected skin twice per day, or as directed by a physician.
HOW SUPPLIED:
Uramaxin (45% Urea) TS
Net wt. 14 oz. (397g) tube, NDC 43538-240-14
Also available:
Uramaxin (45% Urea) Cream
Net wt. 9 oz. (255 g) tube, NDC 43538-210-09
Uramaxin (45% Urea) Nail Gel
28 mL bottle, NDC 43538-200-28
Uramaxin (45% Urea) Lotion
16 fl.oz. (473 mL) bottle, NDC 43538-230-16
Store at controlled room temperature 15o-30oC (59o-86oF).
Protect from freezing.
Manufactured for:
MEDIMETRIKS
PHARMACEUTICALS, INC.
363 Route 46 West. Fairfield, NJ 07004-2402 USA
wwww. medimetriks.com
Manufactured by:
Groupe Parima, Inc. Montreal, QC H4S 1X6 CANADA
| URAMAXIN
TS
urea cream |
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Medimetriks Pharmaceuticals, inc. (019903816) |
| Registrant - Groupe Parima, inc. (252437850) |