KIT FOR THE PREPARATION OF LYMPHOSEEK (TECHNETIUM TC 99M TILMANOCEPT)- tilmanocept
Navidea Biopharmaceuticals, Inc.
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use LYMPHOSEEK safely and effectively. See full prescribing information for LYMPHOSEEK.
Lymphoseek (technetium Tc 99m tilmanocept) injection for subcutaneous, intradermal, subareolar, or peritumoral use Initial U.S. Approval: 2013 RECENT MAJOR CHANGESDosage and Administration, Drug Preparation ( 2.3) 10/2016 INDICATIONS AND USAGELymphoseek is a radioactive diagnostic agent indicated with or without scintigraphic imaging for:
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHSThe Kit for preparation of Lymphoseek contains five Tilmanocept Powder vials each containing 250 mcg tilmanocept, and is packaged either with or without five DILUENT for Lymphoseek vials each containing 4.5 mL of sterile buffered saline with phenol. After radiolabeling with technetium Tc 99m and dilution, Lymphoseek contains approximately 92.5 MBq (2.5 mCi) and 250 mcg of technetium Tc 99m tilmanocept in 0.5 mL to 5 mL total volume for injection. (3) CONTRAINDICATIONSNone. (4) WARNINGS AND PRECAUTIONSHypersensitivity: Ask patients about prior reactions to drugs, especially dextran or modified forms of dextran. Observe for hypersensitivity signs and symptoms following Lymphoseek injection. Have resuscitation equipment and trained personnel immediately available. ( 5.1) ADVERSE REACTIONSThe most common adverse reactions (incidence < 1%) are injection site irritation and/or pain. ( 6.1) To report SUSPECTED ADVERSE REACTIONS, contact Navidea Biopharmaceuticals, Inc. at 1-800-476-5270 or www.lymphoseek.com or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch See 17 for PATIENT COUNSELING INFORMATION. Revised: 1/2017 |
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
Lymphoseek is a radioactive diagnostic agent indicated with or without scintigraphic imaging for:
- Lymphatic mapping using a handheld gamma counter to locate lymph nodes draining a primary tumor site in patients with solid tumors for which this procedure is a component of intraoperative management.
- Guiding sentinel lymph node biopsy using a handheld gamma counter in patients with clinically node negative squamous cell carcinoma of the oral cavity, breast cancer or melanoma.
2 DOSAGE AND ADMINISTRATION
2.1 Radiation Safety – Drug Handling
Lymphoseek is a radioactive drug and should be handled with appropriate safety measures to minimize radiation exposure [see Warnings and Precautions (5.2)] . Use waterproof gloves, effective radiation shielding, and appropriate safety measures when preparing and handling Lymphoseek.
Radiopharmaceuticals should be used by or under the control of physicians who are qualified by specific training and experience in the safe use and handling of radionuclides, and whose experience and training have been approved by the appropriate governmental agency authorized to license the use of radionuclides.
2.2 Recommended Dosing
The recommended dose of Lymphoseek is 18.5 MBq (0.5 mCi) as a radioactivity dose and 50 mcg as a mass dose. Administer Lymphoseek at least 15 minutes prior to initiating intraoperative lymphatic mapping and sentinel node biopsy; complete these procedures within 15 hours after Lymphoseek injection.
Route of Administration and Injection Method
The route of administration depends on the tumor location and the planned injection technique and includes: subcutaneous, intradermal, subareolar, or peritumoral injection.
Lymphoseek may be administered to a patient as a single injection or as multiple injections. The recommended total injection volume for each patient (Table 1) is 0.1 mL administered in a single syringe; 0.5 mL administered in a single syringe or in multiple syringes (0.1 mL to 0.25 mL each); or 1 mL administered in multiple syringes (0.2 mL to 0.5 mL each).
The lymphatic system architecture and function may be changed by prior surgery, radiation, edema, inflammation or metastatic disease, and may result in changes to lymph node localization by a radiopharmaceutical or other tracers, including colorimetric agents. Avoid injections into biopsy wound areas that show evidence of edema or inflammation.
In animal studies, locally injected anesthetics have been reported to reduce lymphatic flow. Concomitant administration of local anesthetics with Lymphoseek is not recommended and may impair the lymph nodal mapping.
2.3 Drug Preparation
General Considerations
- Kit for the preparation of Lymphoseek contains five Tilmanocept Powder vials, each containing 250 mcg of tilmanocept rom which 50 mcg is intended for administration to a patient.
- The Kit for the preparation of Lymphoseek is packaged either with or without five DILUENT for Lymphoseek vials each containing 4.5 mL of sterile buffered saline with phenol.
- The Kit for the preparation of Lymphoseek may also be diluted with pharmacy-available sterile 0.9% sodium chloride injection.
- A diluent is used to dilute Lymphoseek after the radiolabeling procedure. The amount of diluent used varies, depending on the total injection volume and the number of syringes used for each patient.
- The vial components of the Kit for the preparation of Lymphoseek are intended solely for use in the preparation of Lymphoseek. Do not administer the unprepared vial components of the Kit directly to a patient.
- Follow aseptic procedures during preparation and administration.
Drug Preparation Instructions
Prior to preparation of Lymphoseek, determine the planned injection technique and the number of injections that will be used for a given patient. For each injection prepare a separate syringe. Based on the planned number of injection syringes and the planned total injection volume per patient, determine (from Table 1 below) the Reconstituted Vial Volume of radiolabeled Lymphoseek.
| Planned Number of Injections for a Patient | Total Injection Volume Per Patient | Reconstituted Vial Volume of Radiolabeled Lymphoseek |
| 1 syringe x 0.1 mL | 0.1 mL | 0.5 mL |
| 5 syringes x 0.1 mL or
2 syringes x 0.25 mL or2 syringes x 0.25 mL or 1 syringe x 0.5 mL1 syringe x 0.5 mL | 0.5 mL | 2.5 mL |
| 5 syringes x 0.2 mL or
4 syringes x 0.25 mL or4 syringes x 0.25 mL or 2 syringes x 0.5 mL2 syringes x 0.5 mL | 1 mL | 5 mL |
Once the Reconstituted Vial Volume is established, use the following steps to prepare radiolabeled Lymphoseek:
Radiolabeling
a. Inspect the Tilmanocept Powder vial for any damage. Do not use if vial integrity appears compromised. Do not vent the Tilmanocept Powder vial prior to or during radiolabeling.
b. Use Technetium Tc 99m pertechnetate, sodium injection solution from a technetium Tc 99m generator within 8 hours of its elution.
c. Using a sterile syringe, aseptically draw approximately 92.5 MBq (2.5 mCi) of Technetium Tc 99m pertechnetate sodium injection solution in either about 0.35 mL volume (for 0.5 mL Reconstituted Vial Volume) or about 0.7 mL volume (for 2.5 mL or 5 mL Reconstituted Vial Volume). Assay the syringe for technetium Tc 99m activity in a dose calibrator.
d. Write the radioactivity amount, the Reconstituted Vial Volume, date and time, expiration time and lot number in the space provided on the radioactive product vial label and affix it to the Tilmanocept Powder vial. Place the vial in a radiation shield and sanitize the septum with alcohol wipe.
e. Aseptically add Technetium Tc 99m pertechnetate, sodium injection solution to the Tilmanocept Powder vial. Without withdrawing the needle, remove an equal volume of headspace gas. Do not vent.
f. Remove the needle, gently shake the vial to mix the contents, and then let it stand at room temperature for at least 15 minutes.
Reconstitution
g. Aseptically add the supplied DILUENT for Lymphoseek or pharmacy-available sterile 0.9% sodium chloride injection to the radiolabeled product in the Tilmanocept Powder vial to bring the volume to the Reconstituted Vial Volume of 0.5 mL, 2.5 mL, or 5 mL prior to filling the patient dose in syringe(s). To normalize pressure, withdraw an equal volume of headspace gas.
h. Each Lymphoseek vial, once radiolabeled and reconstituted, would contain sufficient amount to provide doses for up to four patients when prepared according to the instructions.
Quality Control of Radiolabeled Solution
i. Assay the reconstituted vial for total radioactivity using a dose calibrator. Write the technetium Tc 99m activity concentration, total volume, assay time and date, expiration time, and lot number on the shield label supplied with the Kit. Affix the label to the shield.
j. Determine the radiochemical purity of the radiolabeled product [see Dosage and Administration (2.4)] . Do not use if the radiochemical purity is less than 90%.
k. Withdraw the required volume of the radiolabeled product into the required number of syringes. Assay the syringe(s) in a dose calibrator. Write the radioactivity amount, date and time of assay, volume, and expiration time (this is not to exceed 6 hours from preparation time) on the supplied syringe label and affix it to the syringe(s).
Duration of Use and Storage of Radiolabeled Solution
l. Store the radiolabeled Lymphoseek in radiation shielding at room temperature.
m. Use the radiolabeled Lymphoseek within 6 hours of preparation. Discard the unused radiolabeled Lymphoseek.
2.4 Determination of Radiochemical Purity of Radiolabeled Lymphoseek
Determine radiochemical purity of the reconstituted radiolabeled Lymphoseek by Instant Thin Layer Chromatography (ITLC) using either Whatman Grade 1, 3MM, 31ET Chr or Biodex 150-001 Red Strips (cellulose chromatography paper) using the following method:
- Mark the chromatographic strip for origin, mid and solvent front lines with a pencil as shown below:
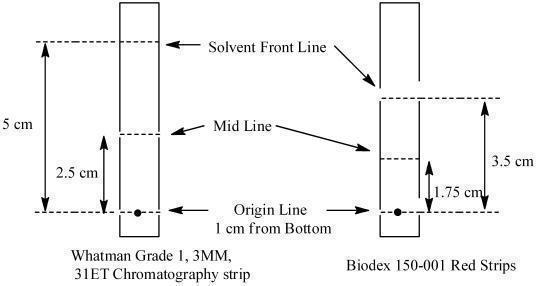
- Apply a small drop (3 - 10 microliters) of the reconstituted product at the center of the origin line chromatography strip. Let the product spot dry.
- Place the strip into a chromatography chamber containing 1 mL of acetone as the developing solvent. Allow the solvent to migrate to the solvent front line (5 cm from the bottom of the Whatman strips and 3.5 cm for the Biodex strip). Remove the strip from the chamber, let it dry and cut it in half. Count each half of the strip with a suitable radioactivity counting apparatus (dose calibrator or multichannel analyzer).
- Calculate the percent radiochemical purity (% RCP) as follows:
% RCP = Counts (activity) in bottom half x 100
Counts (activity) in bottom half + Counts (activity) in top half - Do not use the reconstituted Lymphoseek if the radiochemical purity is less than 90%.
2.5 Lymphatic Mapping and Sentinel Lymph Node Biopsy Following Injection of Lymphoseek
- Lymphoscintigraphy may be used to assist in planning the lymph node mapping procedures. In clinical studies, preoperative scintigraphic imaging was performed using planar imaging techniques and/or SPECT/CT to establish a map of nodal basins and to facilitate intraoperative identification of lymph nodes. Imaging was performed as early as immediately after injection and up to 21 hours [see Clinical Studies (14)] .
- Use a handheld gamma counter to identify nodes that concentrated the injected radioactivity.
- For intraoperative lymphatic mapping, first measure the background radioactivity counts from tissue at least 20 centimeters distal to the injection site. The three sigma threshold (background radioactivity counts plus three times the square root of the mean background count) may be used as an estimate of the threshold for positive localization of Lymphoseek, as exemplified in Table 2.
| a Average of three 2-second counts or one 10-second count | |
| Background Count a (cpm) | Threshold Value (cpm) |
| 5 | 12 |
| 10 | 20 |
| 15 | 27 |
| 20 | 34 |
| 25 | 40 |
| 30 | 47 |
| 35 | 53 |
| 40 | 59 |
- Lymphoseek is intended to supplement palpation, visual inspection, and other procedures important to lymph node mapping and sentinel node biopsy. Intraoperative lymphatic mapping and sentinel node biopsy using gamma detection of Lymphoseek within lymph nodes should be initiated no sooner than 15 minutes following injection. In clinical studies of breast cancer and melanoma, patients also received a concomitant blue dye tracer for comparative detection of lymph nodes. While most lymph nodes were detected with Lymphoseek, some were detected only with the blue dye tracer or only with palpation [see Clinical Studies (14)] .
2.6 Radiation Dosimetry
The radiation doses to organs and tissues of a patient weighing 70 kg given 18.5 MBq (0.5 mCi) of Lymphoseek are shown in Table 3.
| a Calculated from data of 18 patients with breast cancer who received four peritumoral injections of 4 mcg, 20 mcg, and 100 mcg doses of Lymphoseek. | ||
| b Calculated from data of 18 patients with melanoma who received four intradermal injections of 20 mcg, 100 mcg, and 200 mcg doses of Lymphoseek. Due to the differences in injection sites among patients with melanoma, the injection site was assumed to be the breast for the purposes of this calculation, as it represents the nearest anatomical construct for the skin from the anatomical sites appropriately included in the estimates. | ||
| Target Organ | Breast Cancer
a
mGy (rad) | Melanoma
b
mGy (rad) |
| brain | 0.003 (0.0003) | 0.0927 (0.0093) |
| breast (injection site) | 1.659 (0.1659) | 0.7903 (0.079) |
| gall bladder wall | 0.0349 (0.0035) | 0.0712 (0.0071) |
| lower large intestine wall | 0.0123 (0.0012) | 0.057 (0.0057) |
| small intestine | 0.0101 (0.001) | 0.0594 (0.0059) |
| stomach | 0.0184 (0.0018) | 0.0562 (0.0056) |
| upper large intestine wall | 0.0125 (0.0012) | 0.0582 (0.0058) |
| kidney | 0.1863 (0.0186) | 0.278 (0.0278) |
| liver | 0.0324 (0.0032) | 0.0929 (0.0093) |
| lungs | 0.0374 (0.0037) | 0.0599 (0.006) |
| muscle | 0.0092 (0.0009) | 0.0451 (0.0045) |
| ovaries | 0.187 (0.0187) | 0.2991 (0.0299) |
| red marrow | 0.0127 (0.0013) | 0.0507 (0.0051) |
| bone | 0.0177 (0.0018) | 0.0878 (0.0088) |
| spleen | 0.0285 (0.0029) | 0.0598 (0.006) |
| testes | 0.0501 (0.005) | 0.1043 (0.0104) |
| thymus | 0.1168 (0.0117) | 0.0577 (0.0058) |
| thyroid | 0.088 (0.0088) | 0.0464 (0.0046) |
| urinary bladder | 0.0586 (0.0059) | 0.1401 (0.014) |
| total body | 0.0195 (0.0019) | 0.0547 (0.0055) |
| Effective Dose Equivalent
males females | microSv
296 330.2 | microSv
202.4 251.1 |
3 DOSAGE FORMS AND STRENGTHS
The Kit for preparation of Lymphoseek (technetium Tc 99m tilmanocept) injection is supplied as five Tilmanocept Powder vials each containing 250 mcg tilmanocept, and is packaged either with or without five DILUENT for Lymphoseek vials each containing 4.5 mL of sterile buffered saline with phenol. After radiolabeling with technetium Tc 99m, Lymphoseek contains approximately 92.5 MBq (2.5 mCi) and 250 mcg technetium Tc 99m tilmanocept in 0.5 mL to 5 mL total volume.
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Lymphoseek may pose a risk of hypersensitivity reactions due to its chemical similarity to dextran [see Description (11)] . Serious hypersensitivity reactions have been associated with dextran and modified forms of dextran (such as iron dextran drugs).
Before administering Lymphoseek, ask patients about prior hypersensitivity reactions to drugs, especially to dextran and modified forms of dextran. Have resuscitation equipment and trained personnel immediately available at the time of Lymphoseek administration.
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In open label, single arm clinical trials, 553 patients with either breast cancer, malanoma, or squamous cell carcinoma of the oral cavity, skin, and lip received Lymphoseek. No patients experienced serious adverse reactions. Injection site irritation (4 patients; 0.7%) and pain (1 patient; 0.2%) were reported.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C
There are no adequate or well-controlled studies of Lymphoseek in pregnant women. Additionally, animal reproduction studies have not been conducted with technetium Tc 99m tilmanocept. However, all radiopharmaceuticals, including Lymphoseek, have a potential to cause fetal harm. Lymphoseek should be given to a pregnant woman only if the potential benefit justifies the potential risk to the fetus.
8.3 Nursing Mothers
It is not known whether technetium Tc 99m tilmanocept is present in human milk. Based on the half-life of technetium Tc 99m, a nursing woman should pump and discard milk for at least 60 hours (ten half-lives) after administration of Lymphoseek.
8.4 Pediatric Use
Safety and effectiveness of Lymphoseek in patients less than 18 years of age have not been established.
8.5 Geriatric Use
Of the 553 patients enrolled in clinical studies of breast cancer, melanoma, and squamous cell carcinoma (SCC) of oral cavity, skin, and lip, 179 (32%) were aged 65 or older. Review of the clinical data, including evaluation of the frequency of adverse reactions, has not identified differences in safety or efficacy between elderly patients (65 to 90 years of age) and younger patients (18 to 65 years of age).
11 DESCRIPTION
Chemical Characteristics
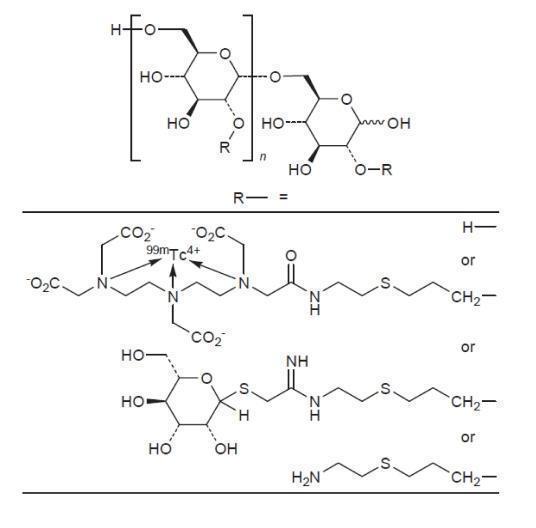
The active ingredient in Lymphoseek, a radioactive diagnostic agent, is technetium Tc 99m tilmanocept. Technetium Tc 99m binds to the diethylenetriaminepentaacetic acid (DTPA) moieties of the tilmanocept molecule.
- Chemically, technetium Tc 99m tilmanocept consists of technetium Tc 99m, dextran 3-[(2-aminoethyl)thio]propyl 17-carboxy-10,13,16-tris(carboxymethyl)-8-oxo-4-thia-7,10,13,16-tetraazaheptadec-1-yl 3-[[2-[[1-imino-2-(D-mannopyranosylthio)ethyl]amino]ethyl]thio]propyl ether complexes.
- The molecular formula of technetium Tc 99m tilmanocept is [C 6H 10O 5] n.(C 19H 28N 4O 9S 99mTc) b.(C 13H 24N 2O 5S 2) c.(C 5H 11NS) a. It contains 3-8 conjugated DTPA (diethylenetriaminepentaacetic acid) molecules (b); 12-20 conjugated mannose molecules (c) with 0-17 amine side chains (a) remaining free.
- The calculated average molecular weight of tilmanocept ranges from 15,281 to 23,454 g/mol.
- Technetium Tc 99m tilmanocept has the following structural formula:
Lymphoseek (technetium Tc 99m tilmanocept) injection is supplied as a Kit which contains five Tilmanocept Powder vials. Each Tilmanocept Powder vial contains the non-radioactive ingredients needed to produce technetium Tc 99m tilmanocept. The vial contains a sterile, non-pyrogenic, white to off-white lyophilized powder (under nitrogen) that consists of a mixture of 250 mcg tilmanocept, 20 mg trehalose dihydrate, 0.5 mg glycine, 0.5 mg sodium ascorbate, and 0.075 mg stannous chloride dihydrate.
The DILUENT for Lymphoseek contains 4.5 mL sterile buffered saline consisting of 0.04% (w/v) potassium phosphate, 0.11% (w/v) sodium phosphate (heptahydrate), 0.5% (w/v) sodium chloride, and 0.4% (w/v) phenol.
Physical Characteristics
Technetium Tc 99m decays by isomeric transition with a physical half-life of approximately 6 hours. The principal photon that is useful for detection and imaging studies is listed in Table 4.
| From: Kocher, D.C. Radioactive decay data tables. DOE/TIC-11026, 108 (1981). | ||
| Radiation | Mean % Disintegration | Mean Energy (keV) |
| Gamma-2 | 89.1 | 140.5 |
External Radiation
The linear mass energy absorption attenuation coefficient for Tc 99m is 18.9 cm -1. The first half-value layer is 0.037 cm of lead (Pb). The use of a 0.25 cm thick standard radiation lead shield will attenuate the radiation emitted by millicurie amounts of technetium Tc 99m by a factor of about 100. A range of values for the relative attenuation of the radiation of technetium Tc 99m that results with various thicknesses of lead shielding are displayed in Table 5.
| Shield Thickness, cm of lead (Pb) | Coefficient of Attenuation |
| 0.037 | 0.5 |
| 0.12 | 10 -1 |
| 0.24 | 10 -2 |
| 0.36 | 10 -3 |
| 0.49 | 10 -4 |
To correct for physical decay of the radionuclide, the fractions that remain at selected intervals after the time of calibration are shown in Table 6.
| Hours | Fraction Remaining |
| 0 | 1 |
| 1 | 0.891 |
| 3 | 0.708 |
| 6 | 0.501 |
| 12 | 0.251 |
| 15 | 0.178 |
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Lymphoseek (technetium Tc 99m tilmanocept) is a radioactive diagnostic agent. It accumulates in lymphatic tissue and selectively binds to mannose binding receptors (CD206) located on the surface of macrophages and dendritic cells. Technetium Tc 99m tilmanocept is a macromolecule consisting of multiple units of diethylenetriaminepentaacetic acid (DTPA) and mannose, each covalently attached to a 10 kDa dextran backbone. The mannose acts as a ligand for the receptor, and the DTPA serves as a chelating agent for labeling with technetium Tc 99m.
12.2 Pharmacodynamics
In in vitro studies, technetium Tc 99m tilmanocept exhibited binding to human mannose binding receptors with a primary binding site affinity of K d = 2.76 x 10 -11 M.
In clinical studies, technetium Tc 99m tilmanocept has been detectable in lymph nodes within 10 minutes and up to 30 hours after injection.
12.3 Pharmacokinetics
In dose-ranging clinical studies, injection site clearance rates were similar across all Lymphoseek doses (4 to 200 mcg) with a mean elimination rate constant in the range of 0.222 to 0.396/hr, resulting in a drug half-life at the injection site of 1.8 to 3.1 hours.
The amount of the accumulated radioactive dose in the liver, kidney, and bladder reached a maximum 1 hour post administration of Lymphoseek and was approximately 1% to 2% of the injected dose in each tissue.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies to assess the carcinogenicity potential of tilmanocept have not been conducted. Tilmanocept was not mutagenic in vitro in the Ames bacterial mutation assay and in the in vitro mouse lymphoma test, and was negative in the in vivo micronucleus test in mice.
Studies on reproductive fertility have not been conducted.
14 CLINICAL STUDIES
14.1 Overview of Clinical Studies
The efficacy and safety of Lymphoseek were assessed in three open-label, multicenter, single arm trials of patients with melanoma, breast cancer, or squamous cell carcinoma (SCC) of the oral cavity, skin, and lip (Studies 1, 2, and 3). Prior to the lymph node mapping and sentinel lymph node biopsy procedures, patients had no known regional nodal or metastatic disease by standard clinical staging criteria.
- In Studies 1 and 2, Lymphoseek was evaluated in patients with breast cancer and melanoma. Diagnostic efficacy for lymphatic mapping was determined by the number of histology-confirmed lymph nodes detected by Lymphoseek and/or the blue dye comparator. Lymphoseek was injected into patients at least 15 minutes prior to initiating lymphatic mapping procedures, and preoperative scintigraphic imaging was performed in 91% of patients. Separately, blue dye was injected shortly prior to initiation of the surgery. Lymphatic mapping was performed intraoperatively using a handheld gamma detection probe followed by excision of lymph nodes identified by Lymphoseek, blue dye, or the surgeon’s visual and palpation examination. The resected lymph nodes were evaluated by histopathology. Lymphoseek localization rate in pathology-positive lymph nodes was also determined.
- In Study 1, of 179 patients who received Lymphoseek, 94 (53%) had known or suspected breast cancer and 85 (47%) had known or suspected melanoma. The median age was 59 years (range 20 to 90 years) and most (72%) were women.
- In Study 2, of 153 patients who received Lymphoseek, 77 (50%) had known or suspected breast cancer and 76 (50%) had known or suspected melanoma. The median age was 61 years (range 26 to 88 years) and most (68%) were women.
- In Study 3, Lymphoseek was evaluated primarily in patients with SCC of the oral cavity (T1-T4a, N0, M0). Diagnostic efficacy was determined by the patient level false negative rate of sentinel lymph node detection by Lymphoseek as confirmed by pathologic assessment of all lymph nodes removed during a planned elective neck dissection (END). Lymphoseek was administered at least 15 minutes prior to the scheduled surgery and preoperative scintigraphic imaging was performed in all patients. Lymphatic mapping was performed intraoperatively using a handheld gamma counter followed by excision of sentinel lymph nodes identified by Lymphoseek. Additional lymph nodes were removed during the END based upon tumor location and surgical practice. All resected lymph nodes (sentinel and non-sentinel) were evaluated for histopathology at the local site. Lymphoseek-identified nodes determined negative for cancer were further evaluated by a central pathology laboratory using additional step sectioning and cytokeratin staining.
- Of the 85 patients who received Lymphoseek, 79 (93%) had intraoral SCC and 6 (7%) had cutaneous SCC. The median age was 59 years (range 23 to 87 years) and most (75%) were men.
14.2 Lymphoscintigraphy
An analysis of the three studies was performed to evaluate the agreement in location of lymph nodes identified by scintigraphic imaging and the handheld gamma counter. At least one scintigraphic “hot spot” was identified in 95% of patients imaged; the percentages were similar across tumor types. Overall, there was 84% agreement on a nodal level (when considering all missing observations as disagreement, as worst case scenario) between the location of preoperative scintigraphic imaging hot spots and the intraoperative lymph node findings (Table 7). Missing observations took the following form: 43 hot spots without corresponding hot nodes and 31 hot nodes without corresponding hot spots.
| * Denominator equals total number of hot spots and/or hot nodes. Numerator equals the numbers where hot spots and hot nodes agreed in location. | ||||
| ** 95% Confidence Intervals | ||||
| Melanoma | Breast Cancer | Head and Neck Cancer | Overall Results | |
| Agreement of Hot Spot and Hot Node Location* |
182/206; 88% (83%, 93%)** |
116/147; 79% (70%, 88%)** |
95/115; 83% (76%, 90%)** |
393/468; 84% (81%, 87%)** |
14.3 Lymphatic Mapping
In Studies 1 and 2 in melanoma and breast cancer, efficacy analyses were based upon comparisons of the number and proportion of resected lymph nodes that contained a lymph node tracer (Lymphoseek and/or blue dye) or neither tracer. Evaluable lymph nodes were resected from 176 Study 1 patients and 152 Study 2 patients who received Lymphoseek at the dose of 0.5 to 2 mCi in 50 mcg administered 15 minutes to 30 hours prior to surgery. Table 8 shows the distribution of resected lymph nodes by the presence or absence of a tracer. Most of the resected lymph nodes were identified by either Lymphoseek (LS) or blue dye (BD) or both. Significantly more resected lymph nodes were identified by Lymphoseek in comparison to blue dye.
| The percentages may not add to 100% due to rounding. | |||||||
| 95% Confidence Intervals (CI) are based on Exact Binomial and represent the spread in the individual estimates. | |||||||
| Study | Tumor | Nodes
n | BD Present
% (95% CI) | LS Present
% (95% CI) | Only BD
Present % (95% CI) | Only LS
Present % (95% CI) | Neither BD
nor LS Present % (95% CI) |
| One | Melanoma | 187 |
65% (57% , 72%) |
93% (88% , 96%) |
2% (0 , 5%) |
29% (23% , 37%) |
6% (3% , 10%) |
| Breast Cancer | 192 |
70% (63% , 77%) |
89% (83% , 93%) |
7% (4% , 12%) |
26% (20% , 32%) |
4% (2% , 8%) |
|
| Two | Melanoma | 198 |
59% (51% , 66%) |
99% (97% , 100%) |
0 (0 , 2%) |
41% (34% , 48%) |
1% (0 , 3%) |
| Breast Cancer | 181 |
62% (55% , 70%) |
100% (98% , 100%) |
0 (0 , 2%) |
38% (30% , 45%) |
0 (0 , 2%) |
|
In Studies 1 and 2 lymphatic mapping was performed in 328 patients with melanoma or breast cancer. The overall rate of lymph node detection by Lymphoseek at the patient level was 97% (319/328). The average number of lymph nodes detected by Lymphoseek was approximately 2 per patient.
In Study 3 lymphatic mapping was performed in 83 patients with SCC of the oral cavity. The rate of lymph node detection by Lymphoseek at the patient level was 98% (81/83) with a 95% confidence interval of 92% to 100%. The average number of lymph nodes identified was 4 per patient.
14.4 Guiding Sentinel Lymph Node Biopsy
In Study 3 in patients with SCC of the oral cavity (n=79), skin (n=5), and lip (n=1), pathology findings for Lymphoseek-identified nodes (sentinel lymph nodes) were compared to the pathology findings of all other lymph nodes removed during the scheduled elective node dissection to determine the false negative rate of Lymphoseek. Thirty-nine patients were determined to have pathology-positive regional lymph nodes. In these patients, the Lymphoseek false negative rate for detecting patients with cancer-positive nodes was 2.6% (95% CI: 0.06% to 13.5%). In this study the pathology-positive nodes were all found in patients with SCC of the oral cavity.
Supportive analyses were conducted in Studies 1 and 2 in patients with breast cancer or melanoma. The presence or absence of Lymphoseek in nodes resected from patients determined by pathology staging to have lymphatic spread of cancer (n=64) was evaluated. The overall patient level rate for identifying at least one cancer-positive node in these pathology-positive patients (both cancers combined) was 97%. Lymphoseek identified 27 out of 29 node positive breast cancer patients and all of the 35 node positive melanoma patients.
16 HOW SUPPLIED/STORAGE AND HANDLING
The Kit for the preparation of Lymphoseek (technetium Tc 99m tilmanocept) injection (NDC 52579-1604-5) includes:
- Five vials of Tilmanocept Powder, 250 mcg (NDC 52579-1695-1)
- Prescribing information
- Five labels for shields
- Twenty-five labels for product vials and individual syringes
The Kit for the preparation of Lymphoseek (technetium Tc 99m tilmanocept) injection (NDC 52579-1600-5) includes:
- Five vials of Tilmanocept Powder, 250 mcg (NDC 52579-1695-1)
- Five vials of DILUENT for Lymphoseek (NDC 52579-1649-1)
- Prescribing information
- Five labels for shields
- Twenty-five labels for product vials and individual syringes
Storage
Store Kit for the preparation of Lymphoseek (technetium Tc 99m tilmanocept) injection in the original packaging at USP controlled room temperature 20 oC - 25 oC (68 oF - 77 oF), excursions permitted to 15°C to 30°C (59°F to 86°F). Store radiolabeled Lymphoseek in radiation shielding at room temperature.
Use radiolabeled Lymphoseek within 6 hours of preparation.
Handling
This Kit for the preparation of Lymphoseek (technetium Tc 99m tilmanocept) injection is approved for distribution to persons licensed by the U.S. Nuclear Regulatory Commission to use by product material identified in 10 CFR 35.200 or under an equivalent license issued by an Agreement State.
17 PATIENT COUNSELING INFORMATION
- Advise patients to seek medical attention for reactions following injection of Lymphoseek such as difficulty breathing, skin rash, or other allergy manifestations.
- Inform nursing women to pump and discard milk for at least 60 hours following administration of Lymphoseek injection [see Use in Specific Populations (8.3)] .
Distributed by:
Navidea Biopharmaceuticals, Inc.,
Dublin, OH 43017
Lymphoseek is a registered trademark of Navidea Biopharmaceuticals, Inc.
Revision date: 10/2016
6-D001712E
Package/Label - Principal Display Panel - Vial Container (Part 1 - 250 mcg Tilmanocept Powder Vial)
NDC 52579-1695-1
Tilmanocept Powder for preparation of Lymphoseek ® (technetium Tc 99m tilmanocept) injection
250 mcg Tilmanocept per Vial
Administer only after radiolabeling with technetium Tc 99m.
See insert for content, preparation and administration instructions.
Store at controlled room temperature, 20°C - 25°C (68°F - 77°F).
Distributed by:
Navidea Biopharmaceuticals, Inc.
Dublin, OH 43017
Sterile Rx Only
6-L001642F

Package/Label - Principal Display Panel - Vial Container (Part 2 - 4.5 mL DILUENT Vial)
NDC 52579-1649-1
DILUENT for Lymphoseek
For diluting radiolabeled Lymphoseek only
Not for direct administration
See package insert for preparation and administration instructions.
Single Use Vial - Discard unused portion.
RX Only 23001105-N02
Contents: 4.5 mL non-pyrogenic, aqueous solution of 0.04% w/v Potassium Phosphate, 0.11% w/v Sodium Phosphate (Heptahydrate), 0.5% w/v Sodium Chloride and 0.40% w/v Phenol.
Store at controlled room temperature, 20°C - 25°C (68°F - 77°F), in original package, excursions permitted to 15°C to 30°C (59°F to 86°F).
Distributed by:
Navidea Biopharmaceuticals, Inc.
Dublin, OH 43017

Package/Label - Principal Display Panel - Radioassay Information Label (Product and Syringe)
CAUTION RADIOACTIVE MATERIAL
Rx Only
Lymphoseek ® (technetium Tc 99m tilmanocept) injection
Store at controlled room temperature, 20°C - 25°C (68°F - 77°F).
MBq (mCi) Tc 99m/mL
Volume (mL)
Time/Date
Expiration Time Lot No.
6-L001694D
Package/Label - Principal Display Panel - Radioassay Information Label (Shield) for NDC 52579-1600-5
Shield Label for NDC 52579-1600-5
Lymphoseek
® (technetium Tc 99m tilmanocept) injection
CAUTION RADIOACTIVE MATERIAL
Rx Only
Contents:
Technetium Tc 99m
pertechnetate, sodium
Tilmanocept 50 mcg
Trehalose, Dihydrate 4 mg
Glycine 0.1 mg
Sodium Ascorbate 0.1 mg
Stannous Chloride, Dihydrate 0.015 mg
Buffered Saline with phenol q.s.
Store at controlled room temperature,
20°C - 25°C (68°F - 77°F).
Discard Unused Portion
MBq (mCi) Tc 99m/mL
Volume (mL)
Time/Date
Expiration Time Lot No.
6-L001592E
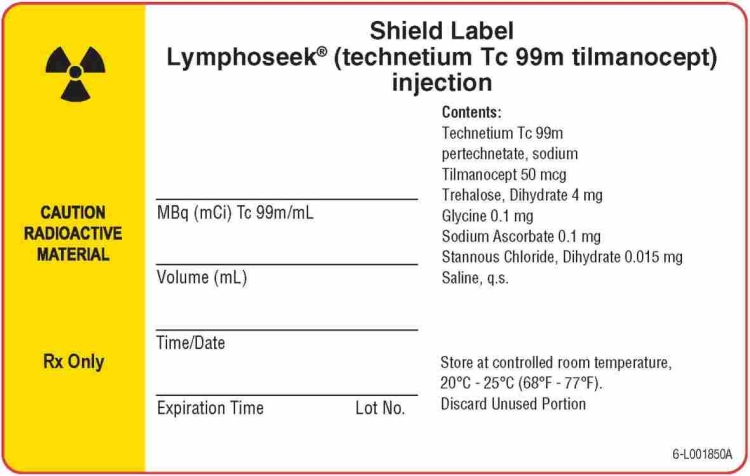
Package/Label - Principal Display Panel - Radioassay Information Label (Shield) for NDC 52579-1604-5
Shield Label for NDC 52579-1604-5
Lymphoseek
® (technetium Tc 99m tilmanocept) injection
CAUTION RADIOACTIVE MATERIAL
Rx Only
Contents:
Technetium Tc 99m
pertechnetate, sodium
Tilmanocept 50 mcg
Trehalose, Dihydrate 4 mg
Glycine 0.1 mg
Sodium Ascorbate 0.1 mg
Stannous Chloride, Dihydrate 0.015 mg
Saline, q.s.
Store at controlled room temperature,
20°C - 25°C (68°F - 77°F).
Discard Unused Portion
MBq (mCi) Tc 99m/mL
Volume (mL)
Time/Date
Expiration Time Lot No.
6-L001850A
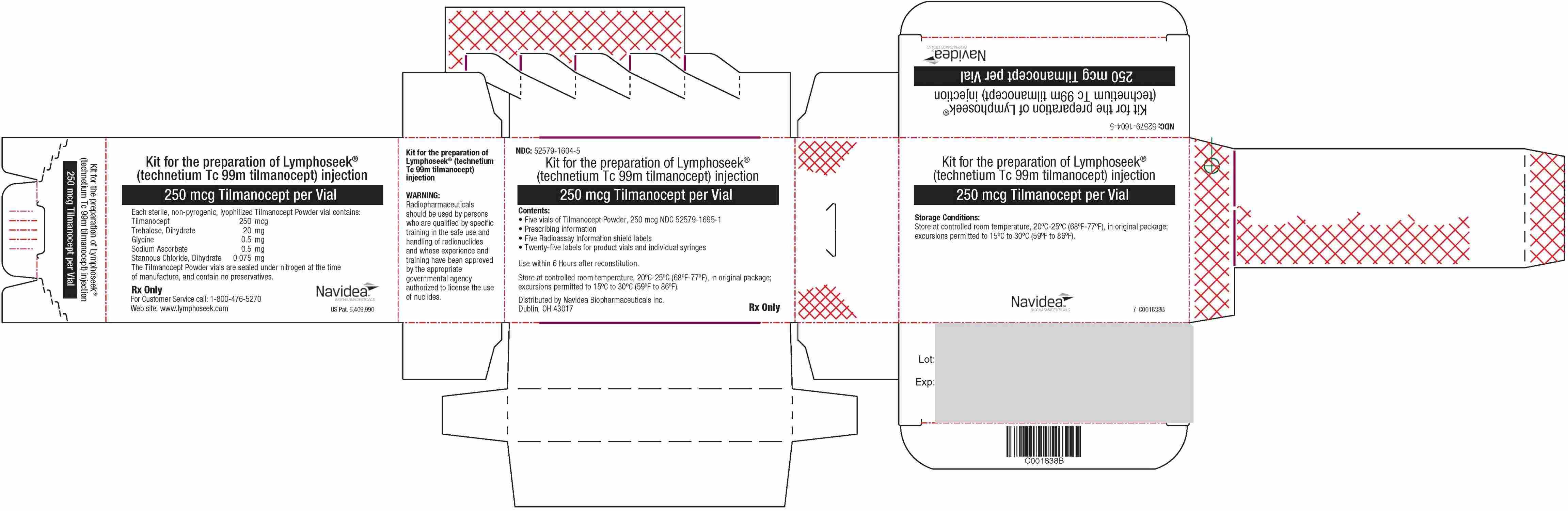
Package/Label - Principal Display Panel - 5 Vial Box With Diluent (NDC 52579-1600-5)
NDC: 52579-1600-5
Kit for the Preparation of Lymphoseek ® (technetium Tc 99m tilmanocept) injection
250 mcg Tilmanocept per Vial
Contents:
- Five vials of Tilmanocept Powder, 250 mcg NDC 52579-1695-1
- Five vials of DILUENT for Lymphoseek NDC 52579-1649-1
- Prescribing information
- Five Radioassay Information shield labels
- Twenty-five labels for product vials and individual syringes
Use within 6 hours after reconstitution.
Storage Conditions:
Store at controlled room temperature, 20°C-25°C (68°F-77°F), in original package; excursions permitted to 15°C to 30°C (59°F to 86°F).
Distributed by Navidea Biopharmaceuticals Inc.
Dublin, OH 43017
Each sterile, non-pyrogenic, lyophilized Tilmanocept Powder vial contains:
Tilmanocept 250 mcg; Trehalose, Dihydrate 20 mg; Glycine 0.5 mg; Sodium Ascorbate 0.5 mg; Stannous Chloride, Dihydrate 0.075 mg
The Tilmanocept Powder vials are sealed under nitrogen at the time of manufacture, and contain no preservatives.
DILUENT for Lymphoseek contains:
Each vial contains 4.5 mL sterile, non-pyrogenic, aqueous solution of:
Potassium Phosphate 0.04% w/v; Sodium Phosphate-7H
2O 0.11% w/v; Sodium Chloride 0.50% w/v; Phenol 0.40% w/v
Rx Only
For Customer Service call: 1-800-476-5270
Web site:
www.lymphoseek.com
WARNING: Radiopharmaceuticals should be used by persons who are qualified by specific training in the safe use and handling of radionuclides and whose experience and training have been approved by the appropriate governmental agency authorized to license the use of nuclides.
7-C001685C

Package/Label - Principal Display Panel - 5 Vial Box Without Diluent (NDC 52579-1604-5)
NDC: 52579-1604-5
Kit for the Preparation of Lymphoseek ® (technetium Tc 99m tilmanocept) injection
250 mcg Tilmanocept per Vial
Contents:
- Five vials of Tilmanocept Powder, 250 mcg NDC 52579-1695-1
- Prescribing information
- Five Radioassay Information shield labels
- Twenty-five labels for product vials and individual syringes
Use within 6 hours after reconstitution.
Storage Conditions:
Store at controlled room temperature, 20°C-25°C (68°F-77°F), in original package; excursions permitted to 15°C to 30°C (59°F to 86°F).
Distributed by Navidea Biopharmaceuticals Inc.
Dublin, OH 43017
Each sterile, non-pyrogenic, lyophilized Tilmanocept Powder vial contains:
Tilmanocept 250 mcg; Trehalose, Dihydrate 20 mg; Glycine 0.5 mg; Sodium Ascorbate 0.5 mg; Stannous Chloride, Dihydrate 0.075 mg
The Tilmanocept Powder vials are sealed under nitrogen at the time of manufacture, and contain no preservatives.
Rx Only
For Customer Service call: 1-800-476-5270
Web site:
www.lymphoseek.com
WARNING: Radiopharmaceuticals should be used by persons who are qualified by specific training in the safe use and handling of radionuclides and whose experience and training have been approved by the appropriate governmental agency authorized to license the use of nuclides.
7-C001838B
| KIT FOR THE PREPARATION OF LYMPHOSEEK (TECHNETIUM TC 99M TILMANOCEPT)
tilmanocept kit |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| KIT FOR THE PREPARATION OF LYMPHOSEEK (TECHNETIUM TC 99M TILMANOCEPT)
tilmanocept kit |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Navidea Biopharmaceuticals, Inc. (131891467) |