CAPMIST DM- dextromethorphan hydrobromide, guaifenesin and pseudoephedrine hydrochloride tablet
Capital Pharmaceutical, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
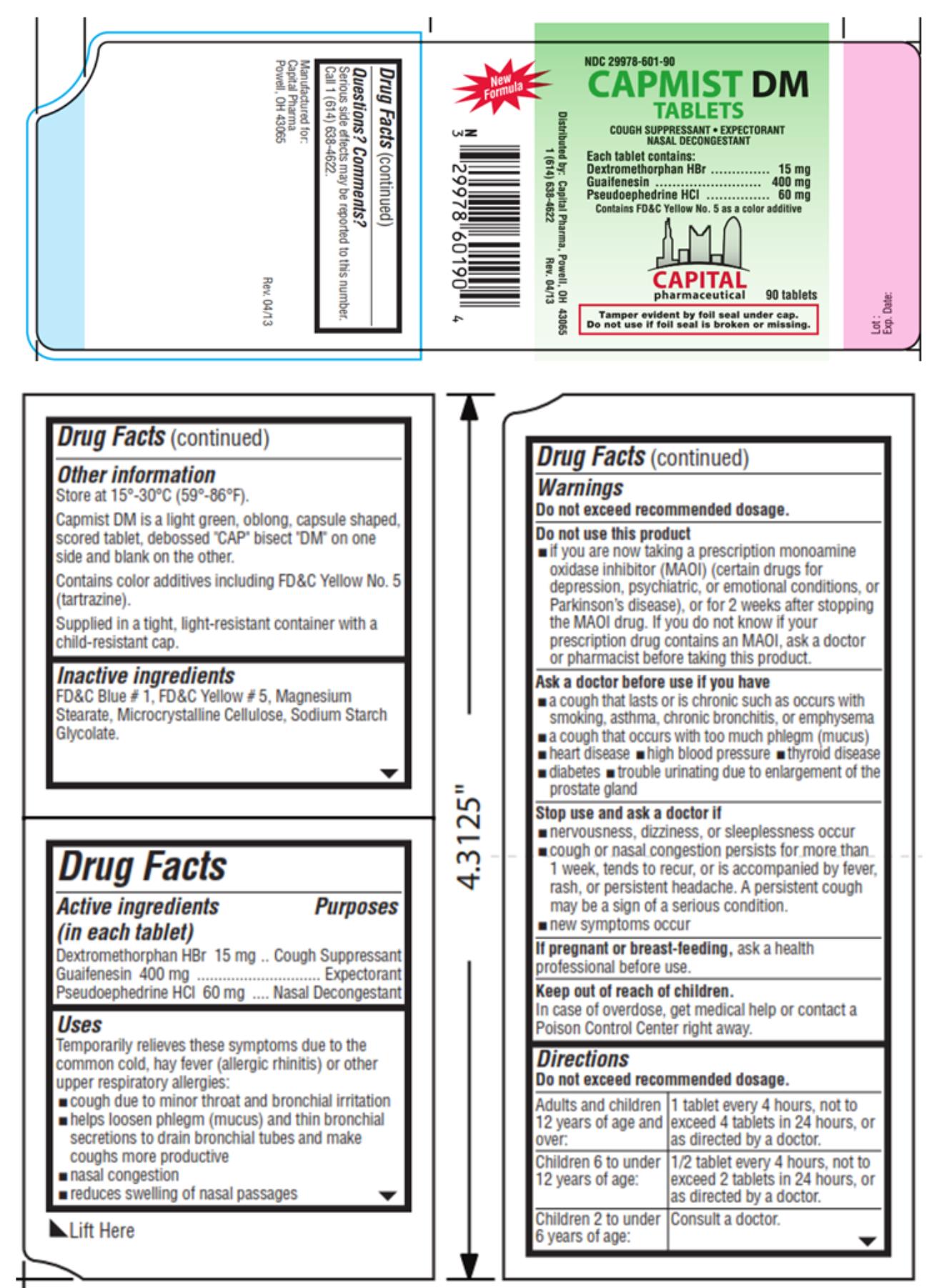
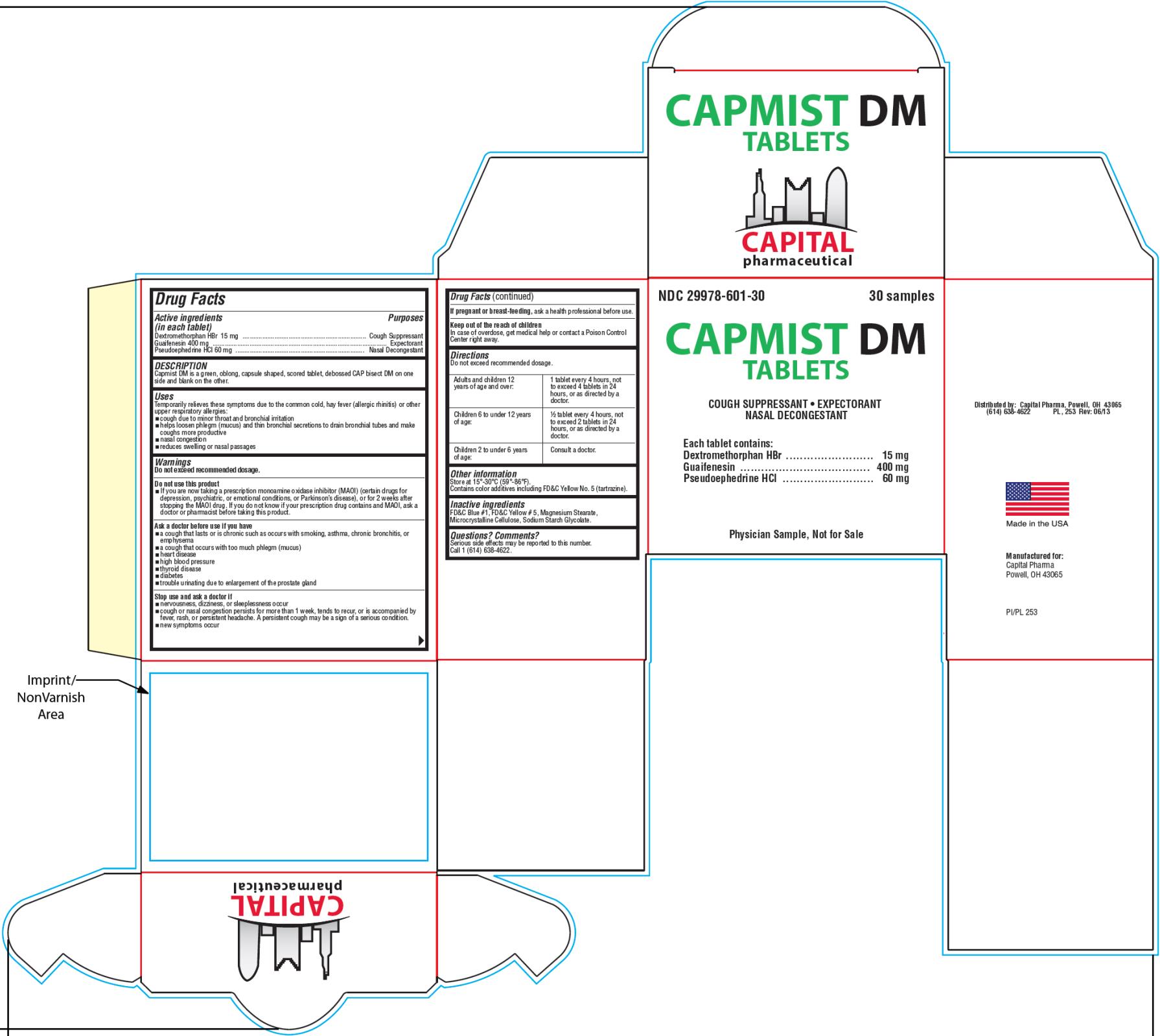
Capmist DM Tablets
Uses
Temporarily relieves these symptoms due to the common cold, hay fever (allergic rhinitis) or other upper respiratory allergies:
- cough due to minor throat and bronchial irritation
- helps loosen phlegm (mucus) and thin bronchial secretions to drain bronchial tubes and make coughs more productive
- nasal congestion
- reduces swelling of nasal passages
Warnings
Do not exceed recommended dosage.
Do not use this product
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- a cough that lasts or is chronic such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- a cough that occurs with too much phlegm (mucus)
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- trouble urinating due to enlargement of the prostate gland
Directions
| Adults and children 12 years of age and over: | 1 tablet every 4 hours, not to exceed 4 tablets in 24 hours, or as directed by a doctor. |
| Children 6 to under 12 years of age: | 1/2 tablet every 4 hours, not to exceed 2 tablets in 24 hours, or as directed by a doctor. |
| Children 2 to under 6 years of age: | Consult a doctor. |
Other information
Store at 15°-30°C (59°-86°F).
Capmist DM is a light green, oblong, capsule shaped, scored tablet, debossed “CAP” bisect “DM” on one side and blank on the other.
Contains color additives including FD&C Yellow No. 5 (tartrazine).
Supplied in a tight, light-resistant container with a child-resistant cap.
Inactive ingredients
FD&C Blue #1, FD&C Yellow #5, Magnesium Stearate, Microcrystalline Cellulose, Sodium Starch Glycolate.
| CAPMIST DM
dextromethorphan hydrobromide, guaifenesin and pseudoephedrine hydrochloride tablet |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Capital Pharmaceutical, LLC (831545541) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| TG United, Inc. | 172837085 | manufacture(29978-601) | |