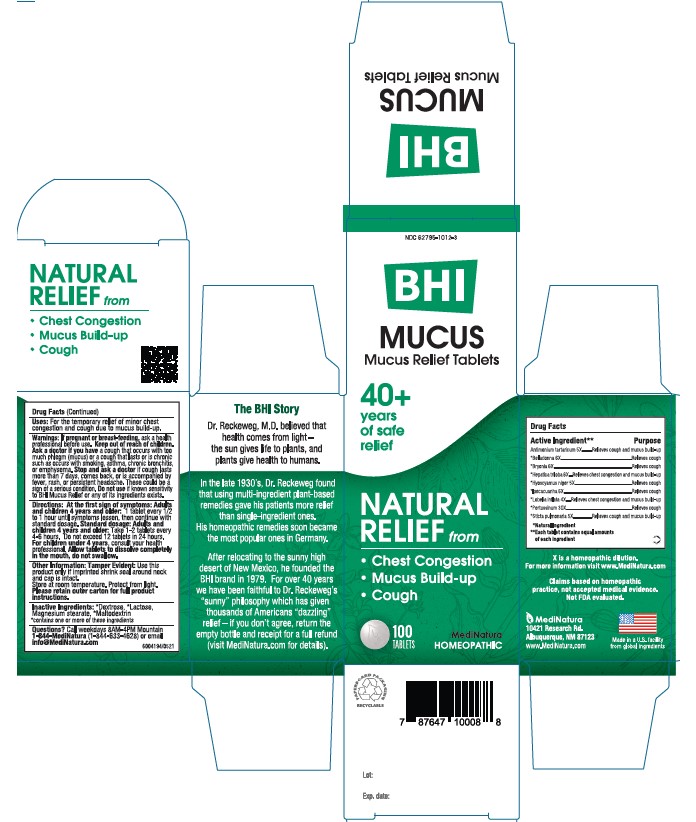
MUCUS RELIEF- antimony potassium tartrate, atropa belladonna, bryonia alba whole, anemone americana, hyoscyamus niger, ipecac, lobelia inflata, human sputum, bordetella pertussis infected, and lobaria pulmonaria tablet
MediNatura Inc
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
BHI Mucus Tablet
ACTIVE INGREDIENT
Each tablet contains:
Antimonium tartaricum 5X, *Belladonna 6X, *Bryonia alba 6X, *Hepatica triloba 6X, *Hyoscyamus niger 5X, *Ipecacuanha 6X, *Lobelia inflata 4X, *Pertussinum 30X, *Sticta pulmonaria 5X 33.3 mg each.
*Natural Ingredients
DIRECTIONS
At first sign of symptoms:
Adults and children 4 years and older: 1 tablet every 1/2 to 1 hour until symptoms lessen, then continue with standard dosage.
Standard dosage: Adults and children 4 years and older: Take 1-2 tablets every 4 to 6 hours. Do not exceed 12 tablets in 24 hours.
For children under 4, consult your health professional.
Allow tablets to dissolve completely in the mouth, do not swallow.
| MUCUS RELIEF
antimony potassium tartrate, atropa belladonna, bryonia alba whole, anemone americana, hyoscyamus niger, ipecac, lobelia inflata, human sputum, bordetella pertussis infected, and lobaria pulmonaria tablet |
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
| Labeler - MediNatura Inc (079324099) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| MediNatura Inc | 102783016 | manufacture(62795-1012) | |