PROFEND NASAL DECOLONIZATION- povidone iodine usp, 10% w/w swab
Professional Disposables International, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Profend Nasal Decolonization Kit
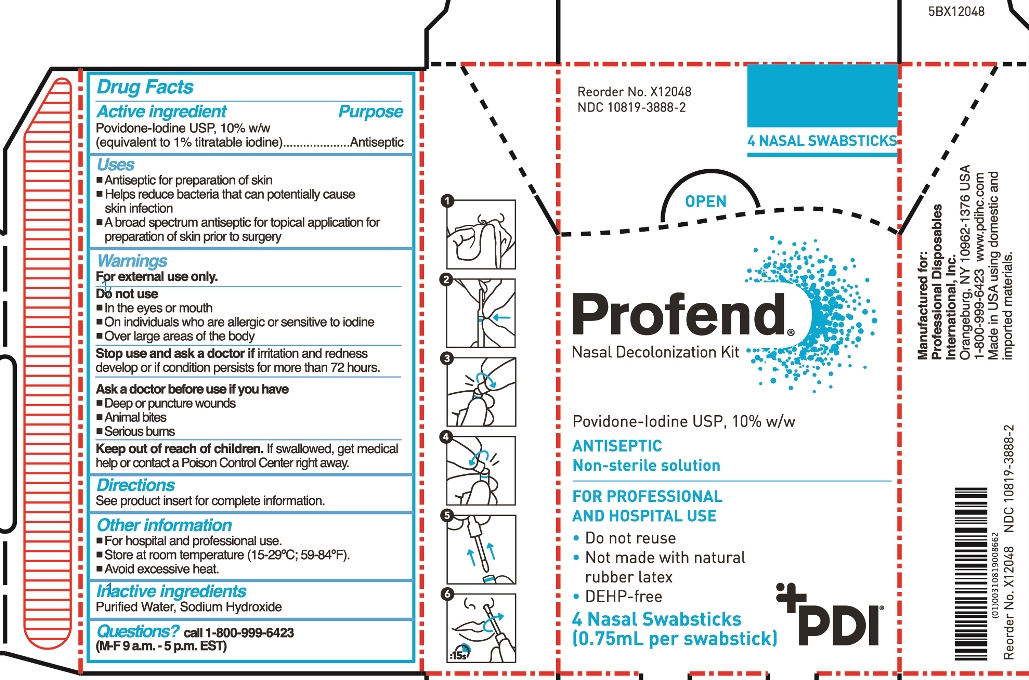
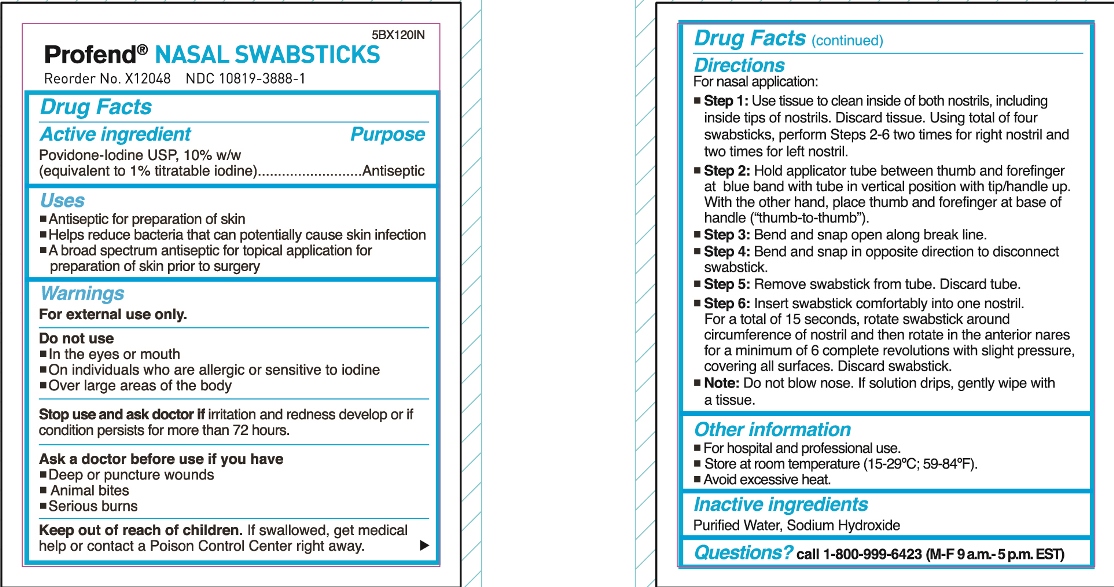
Uses
- Antiseptic for preparation of skin
- Helps reduce bacteria that can potentially cause skin infection
- A broad spectrum antiseptic for topical application for preparation of skin prior to surgery
Warnings
For external use only
Do not use
- in the eyes or mouth
- on individuals who are allergic or sensitive to iodine
- over large areas of the body
Stop use and ask doctor if irritation and redness develop or if condition persists for more than 72 hours.
Ask a doctor before use if you have
- Deep or puncture wounds
- animal bites
- serious burns
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
For nasal application:
Step 1: Use tissue to clean inside of both nostrils, including inside tips of nostrils. Discard tissue.
Using total of four swabsticks, perform Steps 2-6 two times for right nostril and two times for left nostril.
Step 2: Hold applicator tube between thumb and forefinger at blue band with tube in vertical position with tip/handle up. With the other hand, place thumb and forefinger at base of handle (“thumb-to-thumb”).
Step 3: Bend and snap open along break line.
Step 4: Bend and snap in opposite direction to disconnect swabstick.
Step 5: Remove swabstick from tube. Discard tube.
Step 6: Insert swabstick comfortably into one nostril. For a total of 15 seconds, rotate swabstick around circumference of nostril and then rotate in the anterior nares for a minimum of 6 complete revolutions with slight pressure, covering all surfaces. Discard swabstick.
Note: Do not blow nose. If solution drips, gently wipe with a tissue.
| PROFEND NASAL DECOLONIZATION
povidone iodine usp, 10% w/w swab |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Professional Disposables International, Inc. (800777117) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Professional Disposables International, Inc. | 800777117 | manufacture(10819-3888) | |