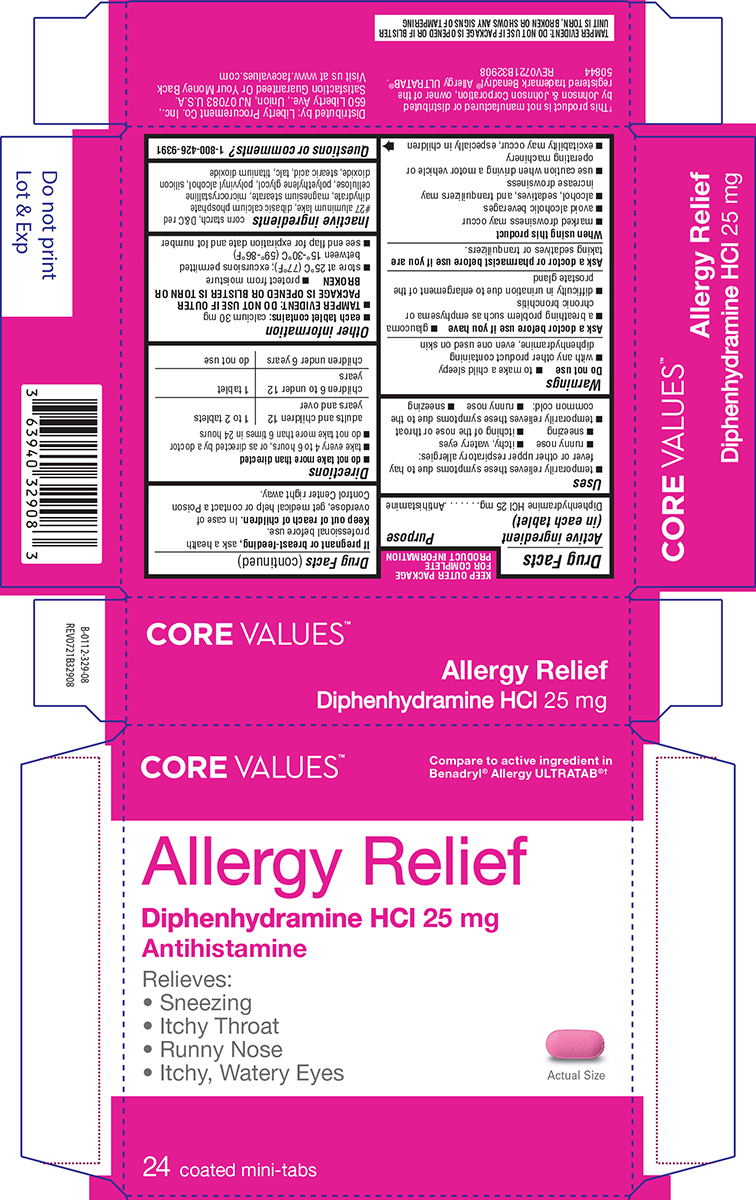
ALLERGY RELIEF- diphenhydramine hcl tablet, film coated
Harmon Store Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Core Values 44-329 Delisted
Uses
- temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- itchy, watery eyes
- sneezing
- itching of the nose or throat
- temporarily relieves these symptoms due to the common cold:
- runny nose
- sneezing
Warnings
Do not use
- to make a child sleepy
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- difficulty in urination due to enlargement of the prostate gland
Directions
- do not take more than directed
- take every 4 to 6 hours, or as directed by a doctor
- do not take more than 6 times in 24 hours
| adults and children 12 years and over | 1 to 2 tablets |
| children 6 to under 12 years | 1 tablet |
| children under 6 years | do not use |
Other information
- each tablet contains: calcium 30 mg
- TAMPER EVIDENT: DO NOT USE IF OUTER PACKAGE IS OPENED OR BLISTER IS TORN OR BROKEN
- store at 25ºC (77ºF); excursions permitted between 15º-30ºC (59º-86ºF)
- protect from moisture
- see end flap for expiration date and lot number
Inactive ingredients
corn starch, D&C red #27 aluminum lake, dibasic calcium phosphate dihydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, silicon
dioxide, stearic acid, talc, titanium dioxide
Principal Display Panel
CORE VALUES™
Compare to active ingredient in
Benadryl® Allergy ULTRATAB®†
Allergy Relief
Diphenhydramine HCl 25 mg
Antihistamine
Relieves:
• Sneezing
• Itchy Throat
• Runny Nose
• Itchy, Watery Eyes
Actual Size
24 coated mini-tabs
†This product is not manufactured or distributed
by Johnson & Johnson Corporation, owner of the
registered trademark Benadryl® Allergy ULTRATAB®.
50844 REV0721B32908
Distributed by: Liberty Procurement Co. Inc.,
650 Liberty Ave., Union NJ 07083 U.S.A.
Satisfaction Guaranteed Or Your Money Back
Visit us at www.facevalues.com
TAMPER EVIDENT: DO NOT USE IF PACKAGE IS OPENED OR IF BLISTER
UNIT IS TORN, BROKEN OR SHOWS ANY SIGNS OF TAMPERING
Core Values 44-329 REV0721B
| ALLERGY RELIEF
diphenhydramine hcl tablet, film coated |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Harmon Store Inc. (804085293) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 038154464 | pack(63940-329) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867837 | manufacture(63940-329) , pack(63940-329) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867894 | manufacture(63940-329) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 868734088 | manufacture(63940-329) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 967626305 | pack(63940-329) | |