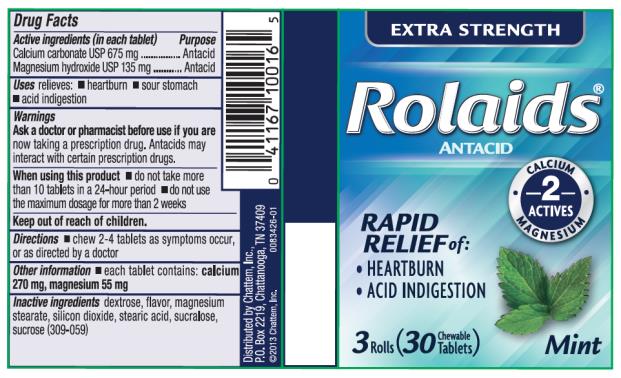
Label: ROLAIDS EXTRA STRENGTH MINT- calcium carbonate and magnesium hydroxide tablet, chewable
-
NDC Code(s):
41167-1001-0,
41167-1001-1,
41167-1001-3,
41167-1001-5, view more41167-1001-6
- Packager: Chattem, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 23, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Use
- Warnings
- Directions
- Other information
- Inactive ingredients
- Active ingredient
- Purpose
- Use
- Warnings
- Directions
- Other information
-
Inactive ingredients
dextrose, flavor, magnesium stearate, silicon dioxide, stearic acid, sucralose, sucrose (309-054)
As a daily source of calcium and magnesium, chew 2 tablets twice daily.
Supplement Facts
Serving Size: 2 Tablets, Servings Per Container: 48, Amount Per Serving: Calories 8, Total Carb 2 g (<1% DV*), Sugars 2 g (†DV), Calcium 540 mg (54% DV), Magnesium 110 mg (29% DV). *Percent Daily Values (DV) are based on a 2,000 calories diet.
†Daily Value not established. - Other ingredients
- Active ingredient
- Purpose
- Use
- Warnings
- Directions
- Other information
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ROLAIDS EXTRA STRENGTH MINT
calcium carbonate and magnesium hydroxide tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:41167-1001 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM CARBONATE (UNII: H0G9379FGK) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CARBONATE 675 mg MAGNESIUM HYDROXIDE (UNII: NBZ3QY004S) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM HYDROXIDE 135 mg Inactive Ingredients Ingredient Name Strength DEXTROSE (UNII: IY9XDZ35W2) MAGNESIUM STEARATE (UNII: 70097M6I30) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STEARIC ACID (UNII: 4ELV7Z65AP) SUCRALOSE (UNII: 96K6UQ3ZD4) SUCROSE (UNII: C151H8M554) MALTODEXTRIN (UNII: 7CVR7L4A2D) Product Characteristics Color white Score no score Shape ROUND Size 16mm Flavor MINT Imprint Code R;X Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:41167-1001-6 3 in 1 CELLO PACK 09/01/2013 03/31/2016 1 NDC:41167-1001-5 10 in 1 PACKAGE; Type 0: Not a Combination Product 2 NDC:41167-1001-1 96 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 09/01/2013 3 NDC:41167-1001-3 116 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 09/01/2013 03/31/2019 4 NDC:41167-1001-0 120 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 09/01/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M001 09/01/2013 Labeler - Chattem, Inc. (003336013)