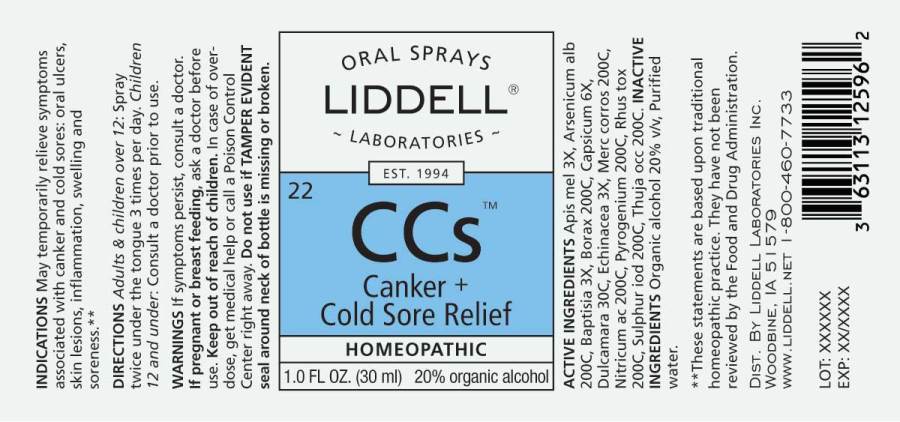
CANKER PLUS COLD SORE RELIEF- apis mellifica, arsenicum album, baptisia tinctoria, borax, capsicum annuum, dulcamara, echinacea (angustifolia), mercurius corrosivus, nitricum acidum, pyrogenium, rhus tox, sulphur iodatum, thuja occidentalis spray
Liddell Laboratories, Inc.
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Drug Facts:
ACTIVE INGREDIENTS:
Apis Mellifica 3X, Arsenicum Album 200C, Baptisia Tinctoria 3X, Borax 200C, Capsicum Annuum 6X, Dulcamara 30C, Echinacea (Angustifolia) 3X, Mercurius Corrosivus 200C, Nitricum Acidum 200C, Pyrogenium 200C, Rhus Tox 200C, Sulphur Iodatum 200C, Thuja Occidentalis 200C.
INDICATIONS:
May temporarily relieve symptoms associated with canker and cold sores: oral ulcers, skin lesions, inflammation, swelling and soreness.**
**These statements are based upon traditional homeopathic practice. They have not been reviewed by the Food and Drug Administration.
WARNINGS:
If symptoms persist, consult a doctor.
If pregnant or breast feeding, ask a doctor before use.
Keep out of reach of children. In case of overdose, get medical help or call a Poison Control Center right away.
Do not use if TAMPER EVIDENT seal around neck of bottle is missing or broken.
KEEP OUT OF REACH OF CHILDREN
Keep out of reach of children. In case of overdose, get medical help or call a Poison Control Center right away.
DIRECTIONS:
Adults & children over 12: Spray twice under the tongue 3 times per day. Children 12 and under: Consult a doctor prior to use.
| CANKER PLUS COLD SORE RELIEF
apis mellifica, arsenicum album, baptisia tinctoria, borax, capsicum annuum, dulcamara, echinacea (angustifolia), mercurius corrosivus, nitricum acidum, pyrogenium, rhus tox, sulphur iodatum, thuja occidentalis spray |
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Liddell Laboratories, Inc. (832264241) |
| Registrant - Apotheca Company (844330915) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Apotheca Company | 844330915 | manufacture(50845-0212) , api manufacture(50845-0212) , label(50845-0212) , pack(50845-0212) | |