TANZEUM- albiglutide injection, powder, lyophilized, for solution
GlaxoSmithKline LLC
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use TANZEUM safely and effectively. See full prescribing information for TANZEUM.
TANZEUM (albiglutide) for injection, for subcutaneous use Initial U.S. Approval: 2014 WARNING: RISK OF THYROID C-CELL TUMORSSee full prescribing information for complete boxed warning.
RECENT MAJOR CHANGES
INDICATIONS AND USAGETANZEUM is a GLP-1 receptor agonist indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. (1) Limitations of Use:
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHSFor injection: 30 mg or 50 mg in a single-dose Pen. (3) CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONSAdverse reactions reported in ≥5% of patients treated with TANZEUM and more frequently than in patients on placebo were upper respiratory tract infection, diarrhea, nausea, injection site reaction, cough, back pain, arthralgia, sinusitis, and influenza. (6.1) To report SUSPECTED ADVERSE REACTIONS, contact GlaxoSmithKline at 1-888-825-5249 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch DRUG INTERACTIONSTANZEUM delays gastric emptying. May impact absorption of concomitantly administered oral medications. (7) USE IN SPECIFIC POPULATIONSSee 17 for PATIENT COUNSELING INFORMATION and Medication Guide. Revised: 12/2017 |
FULL PRESCRIBING INFORMATION
WARNING: RISK OF THYROID C-CELL TUMORS
- •
- Carcinogenicity of albiglutide could not be assessed in rodents, but other glucagon-like peptide-1 (GLP-1) receptor agonists have caused thyroid C-cell tumors in rodents at clinically relevant exposures. Human relevance of GLP-1 receptor-agonist-induced C-cell tumors in rodents has not been determined. It is unknown whether TANZEUM causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans [see Warnings and Precautions (5.1), Nonclinical Toxicology (13.1)].
- •
- TANZEUM is contraindicated in patients with a personal or family history of MTC or in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). Counsel patients regarding the potential risk of MTC with the use of TANZEUM and inform them of the symptoms of thyroid tumors (e.g., mass in the neck, dysphagia, dyspnea, persistent hoarseness). Routine monitoring of serum calcitonin or using thyroid ultrasound monitoring is of uncertain value for early detection of MTC in patients treated with TANZEUM [see Contraindications (4.1), Warnings and Precautions (5.1)].
1 INDICATIONS AND USAGE
TANZEUM is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus [see Clinical Studies (14)].
Limitations of Use:
- •
- TANZEUM is not recommended as first-line therapy for patients inadequately controlled on diet and exercise because of the uncertain relevance of the rodent C-cell tumor findings to humans. Prescribe TANZEUM only to patients for whom the potential benefits are considered to outweigh the potential risk [see Warnings and Precautions (5.1)].
- •
- TANZEUM has not been studied in patients with a history of pancreatitis [see Warnings and Precautions (5.2)]. Consider other antidiabetic therapies in patients with a history of pancreatitis.
- •
- TANZEUM is not indicated in the treatment of patients with type 1 diabetes mellitus or for the treatment of patients with diabetic ketoacidosis. TANZEUM is not a substitute for insulin in these patients.
- •
- TANZEUM has not been studied in patients with severe gastrointestinal disease, including severe gastroparesis. The use of TANZEUM is not recommended in patients with pre-existing severe gastrointestinal disease [see Adverse Reactions (6.1)].
- •
- TANZEUM has not been studied in combination with prandial insulin.
2 DOSAGE AND ADMINISTRATION
2.1 Dosage
The recommended dosage of TANZEUM is 30 mg once weekly given as a subcutaneous injection in the abdomen, thigh, or upper arm region. The dosage may be increased to 50 mg once weekly if the glycemic response is inadequate.
TANZEUM may be administered at any time of day without regard to meals. Instruct patients to administer TANZEUM once a week on the same day each week. The day of weekly administration may be changed if necessary as long as the last dose was administered 4 or more days before.
If a dose is missed, instruct patients to administer as soon as possible within 3 days after the missed dose. Thereafter, patients can resume dosing on their usual day of administration. If it is more than 3 days after the missed dose, instruct patients to wait until their next regularly scheduled weekly dose.
2.2 Concomitant Use with an Insulin Secretagogue (e.g., Sulfonylurea) or with Insulin
When initiating TANZEUM, consider reducing the dosage of concomitantly administered insulin secretagogues (e.g., sulfonylureas) or insulin to reduce the risk of hypoglycemia [see Warnings and Precautions (5.3)].
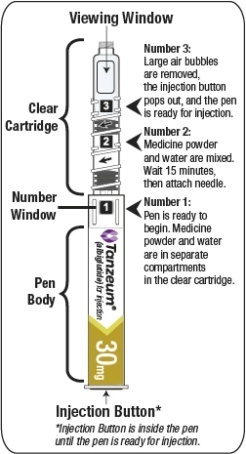
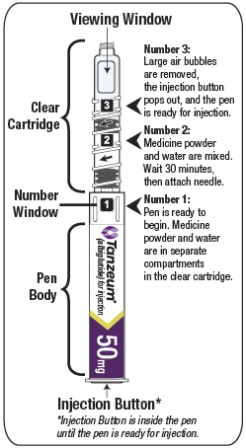
2.3 Reconstitution of the Lyophilized Powder
The lyophilized powder contained within the Pen must be reconstituted prior to administration. See Patient Instructions for Use for complete administration instructions with illustrations. The instructions may also be found at www.TANZEUM.com. Instruct patients as follows:
Pen Reconstitution
- a)
- Hold the Pen body with the clear cartridge pointing up to see the [1] in the number window.
- b)
- To reconstitute the lyophilized powder with the diluent in the Pen, twist the clear cartridge on the Pen in the direction of the arrow until the Pen is felt/heard to “click” into place and the [2] is seen in the number window. This mixes the diluent with the lyophilized powder.
- c)
- Slowly and gently rock the Pen side-to-side 5 times to mix the reconstituted solution of TANZEUM. Advise the patient to not shake the Pen hard to avoid foaming.
- d)
- Wait 15 minutes for the 30-mg Pen and 30 minutes for the 50-mg Pen to ensure that the reconstituted solution is mixed.
Preparing Pen for Injection
- e)
- Slowly and gently rock the Pen side-to-side 5 additional times to mix the reconstituted solution.
- f)
- Visually inspect the reconstituted solution in the viewing window for particulate matter. The reconstituted solution will be yellow in color. After reconstitution, use TANZEUM within 8 hours.
- g)
- Holding the Pen upright, attach the needle to the Pen by pushing it straight down until there is a click and the needle snaps into place. Gently tap the clear cartridge to bring large bubbles to the top.
See Dosage and Administration (2.5) for important administration instructions, including the injection procedure.
Alternate Method of Reconstitution (Healthcare Professional Use Only)
The Patient Instructions for Use provide directions for the patient to wait 15 minutes for the 30-mg Pen and 30 minutes for the 50-mg Pen after the lyophilized powder and diluent are mixed to ensure reconstitution.
Healthcare professionals may utilize the following alternate method of reconstitution. Because this method relies on appropriate swirling and visual inspection of the solution, it should only be performed by healthcare professionals.
- a)
- Follow Step A (Inspect Your Pen and Mix Your Medication) in the Instructions for Use. Make sure you have:
- •
- Inspected the Pen for [1] in the number window and expiration date.
- •
- Twisted the clear cartridge until [2] appears in the number window and a “click” is heard. This combines the medicine powder and liquid in the clear cartridge.
- b)
- Hold the Pen with the clear cartridge pointing up and maintain this orientation throughout the reconstitution.
- c)
- Gently swirl the Pen in small circular motions for at least one minute. Avoid shaking as this can result in foaming, which may affect the dose.
- d)
- Inspect the solution, and if needed, continue to gently swirl the Pen until all the powder is dissolved and you see a clear yellow solution that is free of particles. A small amount of foam, on top of the solution at the end of reconstitution, is normal.
- •
- For 30-mg Pen: Complete dissolution usually occurs within 2 minutes but may take up to 5 minutes, as confirmed by visual inspection for a clear yellow solution free of particles.
- •
- For 50-mg Pen: Complete dissolution usually occurs within 7 minutes but may take up to 10 minutes.
- e)
- After reconstitution, continue to follow the steps in the Instructions for Use, starting at Step B: Attach the Needle.
2.4 Important Administration Instructions
Instruct patients as follows:
- •
- The pen should be used within 8 hours of reconstitution prior to attaching the needle.
- •
- After attaching the supplied needle, remove air bubbles by slowly twisting the Pen until you see the [3] in the number window. At the same time, the injection button will be automatically released from the bottom of the Pen.
- •
- Use immediately after the needle is attached and primed. The product can clog the needle if allowed to dry in the primed needle.
- •
- After subcutaneously inserting the needle into the skin in the abdomen, thigh, or upper arm region, press the injection button. Hold the injection button until you hear a “click” and then hold the button for 5 additional seconds to deliver the full dose.
When using TANZEUM with insulin, instruct patients to administer as separate injections and to never mix the products. It is acceptable to inject TANZEUM and insulin in the same body region but the injections should not be adjacent to each other.
When injecting in the same body region, advise patients to use a different injection site each week. TANZEUM must not be administered intravenously or intramuscularly.
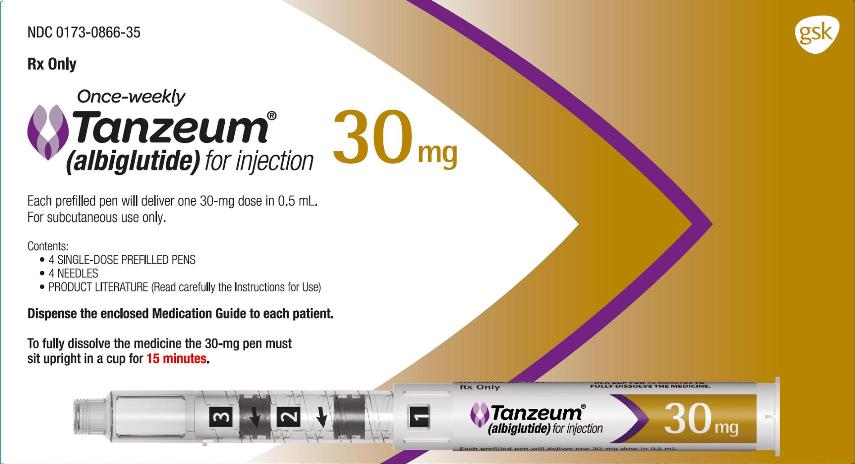
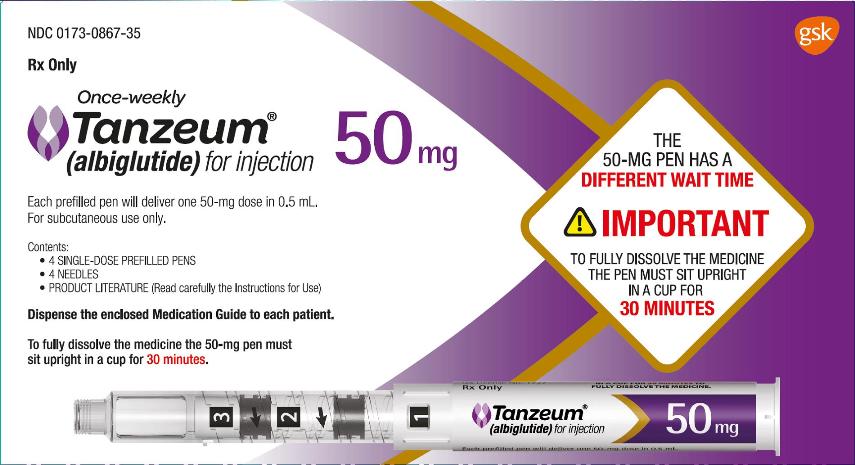
3 DOSAGE FORMS AND STRENGTHS
TANZEUM is supplied as follows:
- •
- For injection: 30-mg lyophilized powder in a single-dose Pen (pen injector) for reconstitution.
- •
- For injection: 50-mg lyophilized powder in a single-dose Pen (pen injector) for reconstitution.
4 CONTRAINDICATIONS
- •
- Medullary Thyroid Carcinoma
TANZEUM is contraindicated in patients with a personal or family history of medullary thyroid carcinoma (MTC) or in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2) [see Warnings and Precautions (5.1)].
- •
- Hypersensitivity
TANZEUM is contraindicated in patients with a prior serious hypersensitivity reaction to albiglutide or to any of the product components. Serious hypersensitivity reactions including angioedema have been reported with TANZEUM [see Warnings and Precautions (5.4)].
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Thyroid C-Cell Tumors
Carcinogenicity of albiglutide could not be assessed in rodents due to the rapid development of drug-clearing, anti-drug antibodies [see Nonclinical Toxicology (13.1)]. Other GLP-1 receptor agonists have caused dose-related and treatment–duration-dependent thyroid C-cell tumors (adenomas or carcinomas) in rodents. Human relevance of GLP-1 receptor-agonist-induced C-cell tumors in rodents has not been determined. It is unknown whether TANZEUM causes thyroid C-cell tumors, including MTC, in humans [see Boxed Warning, Contraindications (4.1)].
Across 8 Phase III clinical trials [see Clinical Studies (14)], MTC was diagnosed in 1 patient receiving TANZEUM and 1 patient receiving placebo. Both patients had markedly elevated serum calcitonin levels at baseline. Cases of MTC in patients treated with liraglutide, another GLP-1 receptor agonist, have been reported in the postmarketing period; the data in these reports are insufficient to establish or exclude a causal relationship between MTC and GLP-1 receptor agonist use in humans.
TANZEUM is contraindicated in patients with a personal or family history of MTC or in patients with MEN 2. Counsel patients regarding the potential risk for MTC with the use of TANZEUM and inform them of symptoms of thyroid tumors (e.g., a mass in the neck, dysphagia, dyspnea, or persistent hoarseness).
Routine monitoring of serum calcitonin or using thyroid ultrasound is of uncertain value for early detection of MTC in patients treated with TANZEUM. Such monitoring may increase the risk of unnecessary procedures, due to the low specificity of serum calcitonin testing for MTC and a high background incidence of thyroid disease. Significantly elevated serum calcitonin may indicate MTC and patients with MTC usually have calcitonin values >50 ng/L. If serum calcitonin is measured and found to be elevated, the patient should be further evaluated. Patients with thyroid nodules noted on physical examination or neck imaging should also be further evaluated.
5.2 Acute Pancreatitis
In clinical trials, acute pancreatitis has been reported in association with TANZEUM.
Across 8 Phase III clinical trials [see Clinical Studies (14)], pancreatitis adjudicated as likely related to therapy occurred more frequently in patients receiving TANZEUM (6 of 2,365 [0.3%]) than in patients receiving placebo (0 of 468 [0%]) or active comparators (2 of 2,062 [0.1%]).
After initiation of TANZEUM, observe patients carefully for signs and symptoms of pancreatitis (including persistent severe abdominal pain, sometimes radiating to the back and which may or may not be accompanied by vomiting). If pancreatitis is suspected, promptly discontinue TANZEUM. If pancreatitis is confirmed, TANZEUM should not be restarted.
TANZEUM has not been studied in patients with a history of pancreatitis to determine whether these patients are at increased risk for pancreatitis. Consider other antidiabetic therapies in patients with a history of pancreatitis.
5.3 Hypoglycemia with Concomitant Use of Insulin Secretagogues or Insulin
The risk of hypoglycemia is increased when TANZEUM is used in combination with insulin secretagogues (e.g., sulfonylureas) or insulin. Therefore, patients may require a lower dose of sulfonylurea or insulin to reduce the risk of hypoglycemia in this setting [see Dosage and Administration (2.2), Adverse Reactions (6.1)].
5.4 Hypersensitivity Reactions
Serious hypersensitivity reactions (including angioedema and generalized pruritus and rash with dyspnea) have been reported with TANZEUM. If hypersensitivity reactions occur, discontinue use of TANZEUM; treat promptly per standard of care, and monitor until signs and symptoms resolve. Do not use in patients with a previous hypersensitivity reaction to TANZEUM [see Contraindications (4)].
Anaphylaxis and angioedema have been reported with other GLP-1 receptor agonists. Use caution in a patient with a history of anaphylaxis or angioedema with another GLP-1 receptor agonist because it is unknown whether such patients will be predisposed to these reactions with TANZEUM.
5.5 Acute Kidney Injury
There have been postmarketing cases of worsening renal function and acute kidney injury in patients treated with TANZEUM, some of which required hemodialysis. Some of the postmarketing events were reported in the absence of gastrointestinal adverse reactions and in patients without known underlying renal disease.
In a trial of TANZEUM in patients with renal impairment [see Clinical Studies (14.3)], the frequency of such gastrointestinal reactions increased as renal function declined [see Use in Specific Populations (8.6)]. Because these reactions may worsen renal function, use caution when initiating or escalating doses of TANZEUM in patients with renal impairment and/or in those reporting severe gastrointestinal symptoms. Advise patients treated with TANZEUM of the potential risk of dehydration in relation to gastrointestinal side effects and to take precautions to avoid fluid depletion [see Use in Specific Populations (8.6)].
6 ADVERSE REACTIONS
The following serious reactions are described below or elsewhere in the prescribing information:
- •
- Risk of Thyroid C-Cell Tumors [see Warnings and Precautions (5.1)]
- •
- Acute Pancreatitis [see Warnings and Precautions (5.2)]
- •
- Hypoglycemia with Concomitant Use of Insulin Secretagogues or Insulin [see Warnings and Precautions (5.3)]
- •
- Hypersensitivity Reactions [see Warnings and Precautions (5.4)]
- •
- Renal Impairment [see Warnings and Precautions (5.5)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Pool of Placebo-Controlled Trials
The data in Table 1 are derived from 4 placebo-controlled trials. TANZEUM was used as monotherapy in 1 trial and as add-on therapy in 3 trials [see Clinical Studies (14)]. These data reflect exposure of 923 patients to TANZEUM and a mean duration of exposure to TANZEUM of 93 weeks. The mean age of participants was 55 years, 1% of participants were 75 years or older and 53% of participants were male. The population in these studies was 48% white, 13% African/African American, 7% Asian, and 29% Hispanic/Latino. At baseline, the population had type 2 diabetes for an average of 7 years and had a mean HbA1c of 8.1%. At baseline, 17% of the population in these studies reported peripheral neuropathy and 4% reported retinopathy. Baseline estimated renal function was normal or mildly impaired (eGFR >60 mL/min/1.73 m2) in 91% of the study population and moderately impaired (eGFR 30 to 60 mL/min/1.73 m2) in 9%.
Table 1 shows common adverse reactions excluding hypoglycemia associated with the use of TANZEUM in the pool of placebo-controlled trials. These adverse reactions were not present at baseline, occurred more commonly on TANZEUM than on placebo, and occurred in at least 5% of patients treated with TANZEUM.
Table 1. Adverse Reactions in Placebo-Controlled Trials Reported in ≥5% of Patients Treated with TANZEUMa
|
Adverse Reaction |
Placebo (n = 468) % |
TANZEUM (n = 923) % |
|
Upper respiratory tract infection |
13.0 |
14.2 |
|
Diarrhea |
10.5 |
13.1 |
|
Nausea |
9.6 |
11.1 |
|
Injection site reactionb |
2.1 |
10.5 |
|
Cough |
6.2 |
6.9 |
|
Back pain |
5.8 |
6.7 |
|
Arthralgia |
6.4 |
6.6 |
|
Sinusitis |
5.8 |
6.2 |
|
Influenza |
3.2 |
5.2 |
- a Adverse reactions reported include those occurring with the use of glycemic rescue medications which included metformin (17% for placebo and 10% for TANZEUM) and insulin (24% for placebo and 14% for TANZEUM).
- b See below for other events of injection site reactions reported.
Gastrointestinal Adverse Reactions: In the pool of placebo-controlled trials, gastrointestinal complaints occurred more frequently among patients receiving TANZEUM (39%) than patients receiving placebo (33%). In addition to diarrhea and nausea (see Table 1), the following gastrointestinal adverse reactions also occurred more frequently in patients receiving TANZEUM: vomiting (2.6% versus 4.2% for placebo versus TANZEUM), gastroesophageal reflux disease (1.9% versus 3.5% for placebo versus TANZEUM), and dyspepsia (2.8% versus 3.4% for placebo versus TANZEUM). Constipation also contributed to the frequently reported reactions. In the group treated with TANZEUM, investigators graded the severity of GI reactions as “mild” in 56% of cases, “moderate” in 37% of cases, and “severe” in 7% of cases. Discontinuation due to GI adverse reactions occurred in 2% of individuals on TANZEUM or placebo.
Injection Site Reactions: In the pool of placebo-controlled trials, injection site reactions occurred more frequently on TANZEUM (18%) than on placebo (8%). In addition to the term “injection site reaction” (see Table 1), the following other types of injection site reactions also occurred more frequently on TANZEUM: injection site hematoma (1.9% versus 2.1% for placebo versus TANZEUM ), injection site erythema (0.4% versus 1.7% for placebo versus TANZEUM), injection site rash (0% versus 1.4% for placebo versus TANZEUM), injection site hypersensitivity (0% versus 0.8% for placebo versus TANZEUM), and injection site hemorrhage (0.6% versus 0.7% for placebo versus TANZEUM). Injection site pruritus also contributed to the frequently reported reactions. The majority of injection site reactions were judged as “mild” by investigators in both groups (73% for TANZEUM versus 94% for placebo). More patients on TANZEUM than on placebo: discontinued due to an injection site reaction (2% versus 0.2%), experienced more than 2 reactions (38% versus 20%), had a reaction judged by investigators to be “moderate” or “severe” (27% versus 6%), and required local or systemic treatment for the reactions (36% versus 11%).
Pool of Placebo- and Active-Controlled Trials
The occurrence of adverse reactions was also evaluated in a larger pool of patients with type 2 diabetes participating in 7 placebo- and active-controlled trials. These trials evaluated the use of TANZEUM as monotherapy, as add-on therapy to oral antidiabetic agents, and as add-on therapy to basal insulin [see Clinical Studies (14)]. In this pool, a total of 2,116 patients with type 2 diabetes were treated with TANZEUM for a mean duration of 75 weeks. The mean age of patients treated with TANZEUM was 55 years, 1.5% of the population in these studies was 75 years or older and 51% of participants were male. Forty-eight percent of patients were white, 15% African/African American, 9% Asian, and 26% were Hispanic/Latino. At baseline, the population had diabetes for an average of 8 years and had a mean HbA1c of 8.2%. At baseline, 21% of the population reported peripheral neuropathy and 5% reported retinopathy. Baseline estimated renal function was normal or mildly impaired (eGFR >60 mL/min/1.73 m2) in 92% of the population and moderately impaired (eGFR 30 to 60 mL/min/1.73 m2) in 8% of the population.
In the pool of placebo- and active-controlled trials, the types and frequencies of common adverse reactions excluding hypoglycemia were similar to those listed in Table 1.
Other Adverse Reactions
Hypoglycemia: The proportion of patients experiencing at least one documented symptomatic hypoglycemic episode on TANZEUM and the proportion of patients experiencing at least one severe hypoglycemic episode on TANZEUM in clinical trials [see Clinical Studies (14)] is shown in Table 2. Hypoglycemia was more frequent when TANZEUM was added to sulfonylurea or insulin [see Warnings and Precautions (5.3)].
Table 2. Incidence (%) of Hypoglycemia in Clinical Trials of TANZEUMa
|
TANZEUM |
||
|
Monotherapyb |
Placebo |
30 mg Weekly |
|
(52 Weeks) |
n = 101 |
n = 101 |
|
Documented symptomaticc |
2% |
2% |
|
Severed |
- |
- |
|
In Combination with Metformin Trial |
Placebo |
TANZEUM |
|
(104 Weeks)e |
n = 101 |
n = 302 |
|
Documented symptomatic |
4% |
3% |
|
Severe |
- |
- |
|
In Combination with Pioglitazone ± |
Placebo |
TANZEUM |
|
Metformin (52 Weeks) |
n = 151 |
n = 150 |
|
Documented symptomatic |
1% |
3% |
|
Severe |
- |
1% |
|
In Combination with Metformin and |
Placebo |
TANZEUM |
|
Sulfonylurea (52 Weeks) |
n = 115 |
n = 271 |
|
Documented symptomatic |
7% |
13% |
|
Severe |
- |
0.4% |
|
In Combination with |
Insulin Lispro |
TANZEUM |
|
Insulin Glargine (26 Weeks) |
n = 281 |
n = 285 |
|
Documented symptomatic |
30% |
16% |
|
Severe |
0.7% |
- |
|
In Combination with |
Insulin Glargine |
TANZEUM |
|
Metformin ± Sulfonylurea (52 Weeks) |
n = 241 |
n = 504 |
|
Documented symptomatic |
27% |
17% |
|
Severe |
0.4% |
0.4% |
|
In Combination with OADs in Renal |
Sitagliptin |
TANZEUM |
|
Impairment (26 Weeks) |
n = 246 |
n = 249 |
|
Documented symptomatic |
6% |
10% |
|
Severe |
0.8% |
- |
- OAD = Oral antidiabetic agents.
- a Data presented are to the primary endpoint and include only events occurring on-therapy with randomized medications and excludes events occurring after use of glycemic rescue medications (i.e., primarily metformin or insulin).
- b In this trial, no documented symptomatic or severe hypoglycemia was reported for TANZEUM 50 mg and these data are omitted from the table.
- c Plasma glucose concentration ≤70 mg/dL and presence of hypoglycemic symptoms.
- d Event requiring another person to administer a resuscitative action.
- e Rate of documented symptomatic hypoglycemia for active controls 18% (glimepiride) and 2% (sitagliptin).
Pneumonia: In the pool of 7 placebo- and active-controlled trials, the adverse reaction of pneumonia was reported more frequently in patients receiving TANZEUM (1.8%) than in patients in the all-comparators group (0.8%). More cases of pneumonia in the group receiving TANZEUM were serious (0.4% for TANZEUM versus 0.1% for all comparators).
Atrial Fibrillation/Flutter: In the pool of 7 placebo- and active-controlled trials, adverse reactions of atrial fibrillation (1.0%) and atrial flutter (0.2%) were reported more frequently for TANZEUM than for all comparators (0.5% and 0%, respectively). In both groups, patients with events were generally male, older, and had underlying renal impairment or cardiac disease (e.g., history of arrhythmia, palpitations, congestive heart failure, cardiomyopathy, etc.).
Appendicitis: In the pool of placebo- and active-controlled trials, serious events of appendicitis occurred in 0.3% of patients treated with TANZEUM compared with 0% among all comparators.
Consistent with the high homology of albiglutide with human GLP-1, the majority of patients (approximately 79%) with anti-albiglutide antibodies also tested positive for anti-GLP-1 antibodies; none were neutralizing. A minority of patients (approximately 17%) who tested positive for anti-albiglutide antibodies also transiently tested positive for antibodies to human albumin.
The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, the incidence of antibodies to albiglutide cannot be directly compared with the incidence of antibodies of other products.
Liver Enzyme Abnormalities: In the pool of placebo- and active-controlled trials, a similar proportion of patients experienced at least one event of alanine aminotransferase (ALT) increase of 3-fold or greater above the upper limit of normal (0.9% and 0.9% for all comparators versus TANZEUM). Three subjects on TANZEUM and one subject in the all-comparator group experienced at least one event of ALT increase of 10-fold or greater above the upper limit of normal. In one of the 3 cases an alternate etiology was identified to explain the rise in liver enzyme (acute viral hepatitis). In one case, insufficient information was obtained to establish or refute a drug-related causality. In the third case, elevation in ALT (10 times the upper limit of normal) was accompanied by an increase in total bilirubin (4 times the upper limit of normal) and occurred 8 days after the first dose of TANZEUM. The etiology of hepatocellular injury was possibly related to TANZEUM but direct attribution to TANZEUM was confounded by the presence of gallstone disease diagnosed on ultrasound 3 weeks after the event.
Gamma Glutamyltransferase (GGT) Increase: In the pool of placebo-controlled trials, the adverse event of increased GGT occurred more frequently in the group treated with TANZEUM (0.9% and 1.5% for placebo versus TANZEUM).
Heart Rate Increase: In the pool of placebo-controlled trials, mean heart rate in patients treated with TANZEUM was higher by an average of 1 to 2 bpm compared with mean heart rate in patients treated with placebo across study visits. The long-term clinical effects of the increase in heart rate have not been established [see Warnings and Precautions (5.6)].
6.2 Immunogenicity
Consistent with the potentially immunogenic properties of protein and peptide pharmaceuticals, patients treated with TANZEUM may develop anti-albiglutide antibodies. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, the incidence of antibodies to albiglutide in the studies described below cannot be directly compared with the incidence of antibodies in other studies or to other products.
In the pool of 7 placebo- and active-controlled trials, 116 (5.5%) of 2,098 patients exposed to TANZEUM tested positive for anti-albiglutide antibodies at any time during the trials. None of these antibodies were shown to neutralize the activity of albiglutide in an in vitro bioassay.
6.3 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of TANZEUM. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Angioedema.
7 DRUG INTERACTIONS
TANZEUM did not affect the absorption of orally administered medications tested in clinical pharmacology studies to any clinically relevant degree [see Clinical Pharmacology (12.3)]. However, TANZEUM causes a delay of gastric emptying, and thereby has the potential to impact the absorption of concomitantly administered oral medications. Caution should be exercised when oral medications are concomitantly administered with TANZEUM.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C
There are no adequate and well-controlled studies of TANZEUM in pregnant women. Nonclinical studies have shown reproductive toxicity, but not teratogenicity, in mice treated with albiglutide at up to 39 times human exposure resulting from the maximum recommended dose of 50 mg/week, based on AUC [see Nonclinical Toxicology (13.1, 13.3)]. TANZEUM should not be used during pregnancy unless the expected benefit outweighs the potential risks.
Due to the long washout period for TANZEUM, consider stopping TANZEUM at least 1 month before a planned pregnancy.
There are no data on the effects of TANZEUM on human fertility. Studies in mice showed no effects on fertility [see Nonclinical Toxicology (13.1)]. The potential risk to human fertility is unknown.
8.3 Nursing Mothers
There are no adequate data to support the use of TANZEUM during lactation in humans.
It is not known if TANZEUM is excreted into human milk during lactation. Given that TANZEUM is an albumin-based protein therapeutic, it is likely to be present in human milk. Decreased body weight in offspring was observed in mice treated with TANZEUM during gestation and lactation [see Nonclinical Toxicology (13.3)]. A decision should be made whether to discontinue nursing or to discontinue TANZEUM, taking into account the importance of the drug to the mother and the potential risks to the infant.
8.4 Pediatric Use
Safety and effectiveness of TANZEUM have not been established in pediatric patients (younger than 18 years).
8.5 Geriatric Use
Of the total number of patients (N = 2,365) in 8 Phase III clinical trials who received TANZEUM, 19% (n = 444) were 65 years and older, and <3% (n = 52) were 75 years and older. No overall differences in safety or effectiveness were observed between these patients and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
8.6 Renal Impairment
Of the total number of patients (N = 2,365) in 8 Phase III clinical trials who received TANZEUM, 54% (n = 1,267) had mild renal impairment (eGFR 60 to 89 mL/min/1.73 m2), 12% (n = 275) had moderate renal impairment (eGFR 30 to 59 mL/min/1.73 m2), and 1% (n = 19) had severe renal impairment (eGFR 15 to <30 mL/min/1.73 m2).
No dosage adjustment is required in patients with mild (eGFR 60 to 89 mL/min/1.73 m2), moderate (eGFR 30 to 59 mL/min/1.73 m2), or severe (eGFR 15 to <30 mL/min/1.73 m2) renal impairment.
Efficacy of TANZEUM in patients with type 2 diabetes and renal impairment is described elsewhere [see Clinical Studies (14.3)]. There is limited clinical experience in patients with severe renal impairment (19 subjects). The frequency of GI events increased as renal function declined. For patients with mild, moderate, or severe impairment, the respective event rates were: diarrhea (6%, 13%, 21%), nausea (3%, 5%, 16%), and vomiting (1%, 2%, 5%). Therefore, caution is recommended when initiating or escalating doses of TANZEUM in patients with renal impairment and/or in those reporting severe gastrointestinal symptoms [see Warnings and Precautions (5.5), Clinical Pharmacology (12.3)].
10 OVERDOSAGE
No data are available with regard to overdosage in humans. Anticipated symptoms of an overdose may be severe nausea, vomiting, and headache.
In the event of an overdose, appropriate supportive treatment should be initiated as dictated by the patient’s clinical signs and symptoms. A prolonged period of observation and treatment for these symptoms may be necessary, taking into account the half-life of TANZEUM (5 days).
11 DESCRIPTION
TANZEUM is a GLP-1 receptor agonist, a recombinant fusion protein comprised of 2 tandem copies of modified human GLP-1 genetically fused in tandem to human albumin. The human GLP-1 fragment sequence 7 – 36 has been modified with a glycine substituted for the naturally-occurring alanine at position 8 in order to confer resistance to dipeptidylpeptidase IV (DPP-IV) mediated proteolysis. The human albumin moiety of the recombinant fusion protein, together with the DPP-IV resistance, extends the half-life allowing once-weekly dosing. TANZEUM has a molecular weight of 72,970 Daltons.
TANZEUM is produced by a strain of Saccharomyces cerevisiae modified to express the therapeutic protein.
TANZEUM 30-mg Pen for injection (for subcutaneous use) contains 40.3 mg lyophilized albiglutide and 0.65 mL Water for Injection diluent designed to deliver a dose of 30 mg in a volume of 0.5 mL after reconstitution.
TANZEUM 50-mg Pen for injection (for subcutaneous use) contains 67 mg lyophilized albiglutide and 0.65 mL Water for Injection diluent designed to deliver a dose of 50 mg in a volume of 0.5 mL after reconstitution.
The lyophilized powder of both dose strengths is white to yellow in color and the solvent is a clear and colorless solution. The reconstituted solution is yellow in color.
Inactive ingredients include 153 mM mannitol, 0.01% (w/w) polysorbate 80, 10 mM sodium phosphate, and 117 mM trehalose dihydrate. TANZEUM does not contain a preservative.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
TANZEUM is an agonist of the GLP-1 receptor and augments glucose-dependent insulin secretion. TANZEUM also slows gastric emptying.
12.2 Pharmacodynamics
TANZEUM lowers fasting glucose and reduces postprandial glucose excursions in patients with type 2 diabetes mellitus. The majority of the observed reduction in fasting plasma glucose occurs after a single dose, consistent with the pharmacokinetic profile of albiglutide. In a Phase II trial in Japanese patients with type 2 diabetes mellitus who received TANZEUM 30 mg, a reduction (22%) in postprandial glucose AUC(0-3 h) was observed at steady state (Week 16) compared with placebo following a mixed meal.
A single dose of TANZEUM 50 mg subcutaneous (SC) did not impair glucagon response to low glucose concentrations.
Gastric Motility
TANZEUM slowed gastric emptying compared with placebo for both solids and liquids when albiglutide 100 mg (2 times the maximum approved dosage) was administered as a single dose in healthy subjects.
Cardiac Electrophysiology
At doses up to the maximum recommended dose (50 mg), TANZEUM does not prolong QTc to any clinically relevant extent.
12.3 Pharmacokinetics
Absorption
Following SC administration of a single 30-mg dose to subjects with type 2 diabetes mellitus, maximum concentrations of albiglutide were reached at 3 to 5 days post-dosing. The mean peak concentration (Cmax) and mean area under the time-concentration curve (AUC) of albiglutide were 1.74 mcg/mL and 465 mcg.h/mL, respectively, following a single dose of 30 mg albiglutide in subjects with type 2 diabetes mellitus. Steady-state exposures are achieved following 4 to 5 weeks of once-weekly administration. Exposures at the 30-mg and 50-mg dose levels were consistent with a dose-proportional increase. Similar exposure is achieved with SC administration of albiglutide in the abdomen, thigh, or upper arm. The absolute bioavailability of albiglutide following SC administration has not been evaluated.
Distribution
The mean estimate of apparent volume of distribution of albiglutide following SC administration is 11 L. As albiglutide is an albumin fusion molecule, plasma protein binding has not been assessed.
Metabolism
Albiglutide is a protein for which the expected metabolic pathway is degradation to small peptides and individual amino acids by ubiquitous proteolytic enzymes. Classical biotransformation studies have not been performed. Because albiglutide is an albumin fusion protein, it likely follows a metabolic pathway similar to native human serum albumin which is catabolized primarily in the vascular endothelium.
Elimination
The mean apparent clearance of albiglutide is 67 mL/h with an elimination half-life of approximately 5 days, making albiglutide suitable for once-weekly administration.
Specific Populations
Age, Gender, Race, and Body Weight: Based on the population pharmacokinetic analysis with data collected from 1,113 subjects, age, gender, race, and body weight had no clinically relevant effect on the pharmacokinetics of albiglutide.
Pediatric Patients: No pharmacokinetic data are available in pediatric patients.
Patients with Renal Impairment: In a population pharmacokinetic analysis including a Phase III trial in patients with mild, moderate, and severe renal impairment, exposures were increased by approximately 30% to 40% in severe renal impairment compared with those observed in type 2 diabetic patients with normal renal function.
Patients with Hepatic Impairment: No clinical trials were conducted to examine the effects of mild, moderate, or severe hepatic impairment on the pharmacokinetics of albiglutide. Therapeutic proteins such as albiglutide are catabolized by widely distributed proteolytic enzymes, which are not restricted to hepatic tissue; therefore, changes in hepatic function are unlikely to have any effect on the elimination of albiglutide.
Drug Interaction Studies
In multiple-dose, drug-drug interaction trials no significant change in systemic exposures of the coadministered drugs were observed, except simvastatin (see Table 3). When albiglutide was coadministered with simvastatin, Cmax of simvastatin and its active metabolite simvastatin acid was increased by approximately 18% and 98%, respectively. In the same trial, AUC of simvastatin decreased by 40% and AUC of simvastatin acid increased by 36%. Clinical relevance of these changes has not been established (see Table 3).
Additionally, no clinically relevant pharmacodynamic effects on luteinizing hormone, follicle-stimulating hormone, or progesterone were observed when albiglutide and a combination oral contraceptive were coadministered. Albiglutide did not significantly alter the pharmacodynamic effects of warfarin as measured by the international normalized ratio (INR).
Table 3. Effect of Albiglutide on Systemic Exposure of Coadministered Drugs
|
Coadministered Drug |
Dose of Coadministered Druga |
Dose of TANZEUM |
Geometric Mean Ratio
|
||
|
Analyte |
AUC (90% CI)b |
Cmax (90% CI) |
|||
|
No dose adjustments of coadministered drug required for the following: |
|||||
|
Simvastatin |
80 mg |
50 mg qw for 5 weeks |
Simvastatin |
0.60 (0.52 – 0.69) |
1.18 (1.02 – 1.38) |
|
Simvastatin acid |
1.36 (1.19 – 1.55) |
1.98 (1.75 – 2.25) |
|||
|
Digoxin |
0.5 mg |
50 mg qw for 5 weeks |
Digoxin |
1.09 (1.01 – 1.18) |
1.11 (0.98 – 1.26) |
|
Oral contraceptivec |
0.035 mg ethinyl estradiol and 0.5 mg norethindrone |
50 mg qw for 4 weeks |
Norethindrone |
1.00 (0.96 – 1.04) |
1.04 (0.98 – 1.10) |
|
Levonorgestrel |
1.09 (1.06 – 1.14) |
1.20 (1.11 – 1.29) |
|||
|
Warfarin |
25 mg |
50 mg qw for 5 weeks |
R-Warfarin |
1.02 (0.98 – 1.07) |
0.94 (0.89 – 0.99) |
|
S-Warfarin |
0.99 (0.95 – 1.03) |
0.93 (0.87 – 0.98) |
|||
- qw = Once weekly.
- a Single dose unless otherwise noted.
- b AUC inf for drugs given as a single dose and AUC 24h for drugs given as multiple doses.
- c Subjects received low-dose oral contraceptive for two 28-day treatment cycles (21 days active/7 days placebo).
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
As albiglutide is a recombinant protein, no genotoxicity studies have been conducted.
Carcinogenicity of albiglutide could not be assessed in rodents due to the rapid development of drug-clearing, anti-drug antibodies. Other GLP-1 receptor agonists have caused thyroid C-cell tumors in rodent carcinogenicity studies. Human relevance of GLP-1 receptor-agonist-induced rodent thyroid C-cell tumors has not been determined.
In a mouse fertility study, males were treated with SC doses of 5, 15, or 50 mg/kg/day for 7 days prior to cohabitation with females and continuing through mating. In a separate fertility study, females were treated with SC doses of 1, 5, or 50 mg/kg/day for 7 days prior to cohabitation with males and continuing through mating. Reductions in estrous cycles were observed at 50 mg/kg/day, a dose associated with maternal toxicity (body weight loss and reduced food consumption). There were no effects on mating or fertility in either sex at doses up to 50 mg/kg/day (up to 39 times clinical exposure based on AUC).
13.3 Reproductive and Developmental Toxicity
In order to minimize the impact of the drug-clearing, anti-drug antibody response, reproductive and developmental toxicity assessments in the mouse were partitioned to limit the dosing period to no more than approximately 15 days in each study.
In pregnant mice given SC doses of 1, 5, or 50 mg/kg/day from gestation Days 1 to 6, there were no adverse effects on early embryonic development through implantation at 50 mg/kg/day (39 times clinical exposure based on AUC).
In pregnant mice given SC doses of 1, 5, or 50 mg/kg/day from gestation Days 6 through 15 (organogenesis), embryo-fetal lethality (post-implantation loss) and bent (wavy) ribs were observed at 50 mg/kg/day (39 times clinical exposure based on AUC), a dose associated with maternal toxicity (body weight loss and reduced food consumption).
Pregnant mice were given SC doses of 1, 5, or 50 mg/kg/day from gestation Days 6 to 17. Offspring of pregnant mice given 50 mg/kg/day (39 times clinical exposure based on AUC), a dose associated with maternal toxicity, had reduced body weight pre-weaning, dehydration and coldness, and a delay in balanopreputial separation.
Pregnant mice were given SC doses of 1, 5, or 50 mg/kg/day from gestation Day 15 to lactation Day 10. Increased mortality and morbidity were seen at all doses (≥1 mg/kg/day) in lactating females in mouse pre- and postnatal development studies. Mortalities have not been observed in previous toxicology studies in non-lactating or non-pregnant mice, nor in pregnant mice. These findings are consistent with lactational ileus syndrome which has been previously reported in mice. Since the relative stress of lactation energy demands is lower in humans than mice and humans have large energy reserves, the mortalities observed in lactating mice are of questionable relevance to humans. The offspring had decreased pre-weaning body weight which reversed post-weaning in males but not females at ≥5 mg/kg/day (2.2 times clinical exposure based on AUC) with no other effects on development. Low levels of albiglutide were detected in plasma of offspring.
Lactating mice were given SC doses of 1, 5, or 50 mg/kg/day from lactation Days 7 to 21 (weaning) under conditions that limit the impact of lactational ileus (increased caloric intake and culling of litters). Doses ≥1 mg/kg/day (exposures below clinical AUC) caused reduced weight gain in the pups during the treatment period.
14 CLINICAL STUDIES
TANZEUM has been studied as monotherapy and in combination with metformin, metformin and a sulfonylurea, a thiazolidinedione (with and without metformin), and insulin glargine (with or without oral anti-diabetic drugs). The efficacy of TANZEUM was compared with placebo, glimepiride, pioglitazone, liraglutide, sitagliptin, insulin lispro, and insulin glargine.
Trials evaluated the use of TANZEUM 30 mg and 50 mg. Five of the 8 trials allowed optional uptitration of TANZEUM from 30 mg to 50 mg if glycemic response with 30 mg was inadequate.
In patients with type 2 diabetes mellitus, TANZEUM produced clinically relevant reduction from baseline in HbA1c compared with placebo. No overall differences in glycemic effectiveness or body weight were observed across demographic subgroups (age, gender, race/ethnicity, duration of diabetes).
14.1 Monotherapy
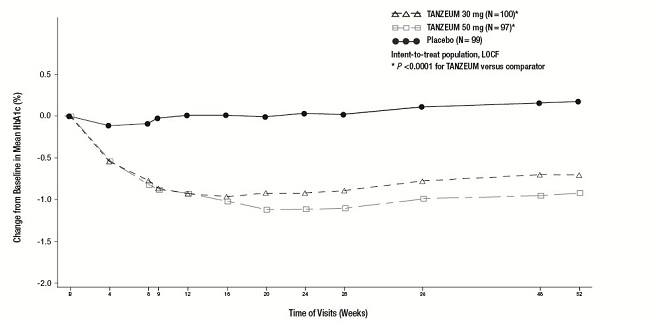
The efficacy of TANZEUM as monotherapy was evaluated in a 52-week, randomized, double-blind, placebo-controlled, multicenter trial. In this trial, 296 patients with type 2 diabetes inadequately controlled on diet and exercise were randomized (1:1:1) to TANZEUM 30 mg SC once weekly, TANZEUM 30 mg SC once weekly uptitrated to 50 mg once weekly at Week 12, or placebo. The mean age of participants was 53 years, 55% of patients were men, the mean duration of diabetes was 4 years, and the mean baseline eGFR was 84 mL/min/1.73 m2. Primary and secondary efficacy results are presented in Table 4. Figure 1 shows the mean adjusted changes in HbA1c from baseline across study visits.
Compared with placebo, treatment with TANZEUM 30 mg or 50 mg resulted in statistically significant reductions in HbA1c from baseline at Week 52 (see Table 4). The adjusted mean change in weight from baseline did not differ significantly between TANZEUM (-0.4 to -0.9 kg) and placebo (-0.7 kg) at Week 52.
Table 4. Results at Week 52 (LOCFa) in a Trial of TANZEUM as Monotherapy
|
Placebo |
TANZEUM 30 mg Weekly |
TANZEUM 50 mg Weekly |
|
|
ITTa (n) |
99 |
100 |
97 |
|
HbA1c (%) | |||
|
Baseline (mean) |
8.0 |
8.1 |
8.2 |
|
Change at Week 52b |
+0.2 |
-0.7 |
-0.9 |
|
Difference from placebob (95% CI) |
-0.8 (-1.1, -0.6)c |
-1.0 (-1.3, -0.8)c |
|
|
Patients (%) achieving HbA1c <7% |
21 |
49 |
40 |
|
FPG (mg/dL) | |||
|
Baseline (mean) |
163 |
164 |
171 |
|
Change at Week 52b |
+18 |
-16 |
-25 |
|
Difference from placebob (95% CI) |
-34 (-46, -22)c |
-43 (-55, -31)c |
- a Intent-to-treat population. Last observation carried forward (LOCF) was used to impute missing data. Data post-onset of rescue therapy are treated as missing. At Week 52, primary efficacy data was imputed for 63%, 34%, and 41% of individuals randomized to placebo, TANZEUM 30 mg, and TANZEUM 50 mg, respectively.
- b Least squares mean adjusted for baseline value and stratification factors.
- c P <0.0001 for treatment difference.
Figure 1. Mean HbA1c Change from Baseline (ITT Population-LOCF) in a Trial of TANZEUM as Monotherapy
14.2 Combination Therapy
Add-On to Metformin
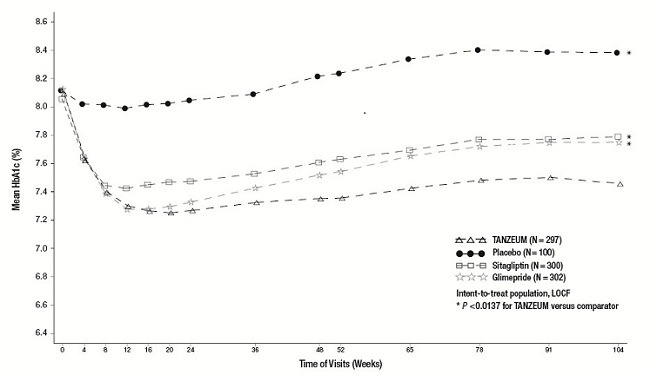
The efficacy of TANZEUM was evaluated in a 104-week randomized, double-blind, multicenter trial in 999 patients with type 2 diabetes mellitus inadequately controlled on background metformin therapy (≥1,500 mg daily). In this trial, TANZEUM 30 mg SC weekly (with optional uptitration to 50 mg weekly after a minimum of 4 weeks) was compared with placebo, sitagliptin 100 mg daily, or glimepiride 2 mg daily (with optional titration to 4 mg daily). The mean age of participants was 55 years, 48% of patients were men, the mean duration of type 2 diabetes was 6 years, and the mean baseline eGFR was 86 mL/min/1.73 m2. Results of the primary and secondary analyses are presented in Table 5. Figure 2 shows the mean adjusted changes in HbA1c across study visits.
Reduction in HbA1c from baseline achieved with TANZEUM was significantly greater than HbA1c reduction achieved with placebo, sitagliptin, and glimepiride at Week 104 (see Table 5). The difference in body weight change from baseline between TANZEUM and glimepiride was significant at Week 104.
Table 5. Results at Week 104 (LOCFa) in a Trial Comparing TANZEUM with Placebo as Add-On Therapy in Patients Inadequately Controlled on Metformin
|
TANZEUM + Metformin |
Placebo + Metformin |
Sitagliptin + Metformin |
Glimepiride + Metformin |
|
|
ITTa (n) |
297 |
100 |
300 |
302 |
|
HbA1c (%) | ||||
|
Baseline (mean) |
8.1 |
8.1 |
8.1 |
8.1 |
|
Change at Week 104b |
-0.6 |
+0.3 |
-0.3 |
-0.4 |
|
Difference from placebo + metforminb (95% CI) |
-0.9 (-1.16, -0.65)c | |||
|
Difference from sitagliptin + metforminb (95% CI) |
-0.4 (-0.53, -0.17)c | |||
|
Difference from glimepiride + metforminb (95% CI) |
-0.3 (-0.45, -0.09)c | |||
|
Proportion achieving HbA1c <7% |
39 |
16 |
32 |
31 |
|
FPG (mg/dL) | ||||
|
Baseline (mean) |
165 |
162 |
165 |
168 |
|
Change at Week 104b |
-18 |
+10 |
-2 |
-8 |
|
Difference from placebo + metforminb (95% CI) |
-28 (-39, -16)c | |||
|
Difference from sitagliptin + metforminb (95% CI) |
-16 (-24, -8)c | |||
|
Difference from glimepiride + metforminb (95% CI) |
-10 (-18, -2)c | |||
|
Body Weight (kg) | ||||
|
Baseline (mean) |
90 |
92 |
90 |
92 |
|
Change at Week 104 b |
-1.2 |
-1.0 |
-0.9 |
+1.2 |
|
Difference from placebo + metforminb (95% CI) |
-0.2 (-1.1, 0.7) | |||
|
Difference from sitagliptin + metforminb (95% CI) |
-0.4 (-1.0, 0.3) | |||
|
Difference from glimepiride + metforminb (95% CI) |
-2.4 (-3.0, -1.7)c |
- a Intent-to-treat population. Last observation carried forward (LOCF) was used to impute missing data. Data post-onset of rescue therapy are treated as missing. At Week 104, primary efficacy data was imputed for 76%, 46%, 55%, and 51% of individuals randomized to placebo, TANZEUM, sitagliptin, and glimepiride, respectively.
- b Least squares mean adjusted for baseline value and stratification factors.
- c P <0.0137 for treatment difference.
Figure 2. Mean HbA1c over Time (ITT Population-LOCF) in a Trial Comparing TANZEUM with Placebo as Add-On Therapy in Patients Inadequately Controlled on Metformin
Add-On to Pioglitazone
The efficacy of TANZEUM was evaluated in a 52-week randomized, double-blind, multicenter trial in 299 patients with type 2 diabetes mellitus inadequately controlled on pioglitazone ≥30 mg daily (with or without metformin ≥1,500 mg daily). Patients were randomized to receive TANZEUM 30 mg SC weekly or placebo. The mean age of participants was 55 years, 60% of patients were men, the mean duration of type 2 diabetes was 8 years, and the mean baseline eGFR was 83 mL/min/1.73 m2. Results of the primary and secondary analyses are presented in Table 6.
Compared with placebo, treatment with TANZEUM resulted in a statistically significant reduction in HbA1c from baseline at Week 52 (see Table 6). The adjusted mean change from baseline in weight did not differ significantly between TANZEUM (+0.3 kg) and placebo (+0.5 kg) at Week 52.
Table 6. Results at Week 52 (LOCFa) in a Trial Comparing TANZEUM with Placebo as Add-On Therapy in Patients Inadequately Controlled on Pioglitazone (with or without Metformin)
|
TANZEUM + Pioglitazone (with or without Metformin) |
Placebo + Pioglitazone (with or without Metformin) |
|
|
ITTa (n) |
150 |
149 |
|
HbA1c (%) | ||
|
Baseline (mean) |
8.1 |
8.1 |
|
Change at Week 52b |
-0.8 |
-0.1 |
|
Difference from placebo + pioglitazoneb (95% CI) |
-0.8 (-0.95, -0.56)c | |
|
Proportion Achieving HbA1c <7% |
44 |
15 |
|
FPG (mg/dL) | ||
|
Baseline (mean) |
165 |
167 |
|
Change at Week 52b |
-23 |
+6 |
|
Difference from placebo + pioglitazoneb (95% CI) |
-30 (-39, -20)c |
- a Intent-to-treat population. Last observation carried forward (LOCF) was used to impute missing data. Data post-onset of rescue therapy are treated as missing. At Week 52, primary efficacy data was imputed for 58% and 32% of individuals randomized to placebo and TANZEUM, respectively.
- b Least squares mean adjusted for baseline value and stratification factors.
- c P <0.0001 for treatment difference.
Add-On to Metformin plus Sulfonylurea
The efficacy of TANZEUM was evaluated in a 52-week randomized, double-blind, multicenter trial in 657 patients with type 2 diabetes mellitus inadequately controlled on metformin (≥1,500 mg daily) and glimepiride (4 mg daily). Patients were randomized to receive TANZEUM 30 mg SC weekly (with optional uptitration to 50 mg weekly after a minimum of 4 weeks), placebo, or pioglitazone 30 mg daily (with optional titration to 45 mg/day). The mean age of participants was 55 years, 53% of patients were men, the mean duration of type 2 diabetes was 9 years, and the mean baseline eGFR was 84 mL/min/1.73 m2. Results of the primary and main secondary analyses are presented in Table 7.
Treatment with TANZEUM resulted in statistically significant reductions in HbA1c from baseline compared with placebo (see Table 7). Treatment with TANZEUM did not meet the pre-specified, non-inferiority margin (0.3%) against pioglitazone. In this trial, TANZEUM provided less HbA1c reduction than pioglitazone and the treatment difference was statistically significant (see Table 7). The change from baseline in body weight for TANZEUM did not differ significantly from placebo but was significantly different compared with pioglitazone (see Table 7).
Table 7. Results at Week 52 (LOCFa) in a Trial Comparing TANZEUM with Placebo as Add-On Therapy in Patients Inadequately Controlled on Metformin plus Sulfonylurea
|
TANZEUM + Metformin + Glimepiride |
Placebo + Metformin + Glimepiride |
Pioglitazone + Metformin + Glimepiride |
|
|
ITTa (n) |
269 |
115 |
273 |
|
HbA1c (%) | |||
|
Baseline (mean) |
8.2 |
8.3 |
8.3 |
|
Change at Week 52b |
-0.6 |
+0.3 |
-0.8 |
|
Difference from placebo + met + glimb (95% CI) |
-0.9 (-1.07, -0.68)c | ||
|
Difference from pioglitazone + met + glimb (95% CI) |
0.25 (0.10, 0.40)d | ||
|
Proportion achieving HbA1c <7% |
30 |
9 |
35 |
|
FPG (mg/dL) | |||
|
Baseline (mean) |
171 |
174 |
177 |
|
Change at Week 52b |
-12 |
+12 |
-31 |
|
Difference from placebo + met + glimb (95% CI) |
-24 (-34, -14)c | ||
|
Difference from pioglitazone + met + glimb (95% CI) |
19 (11, 27)c | ||
|
Body Weight (kg) | |||
|
Baseline (mean) |
91 |
90 |
91 |
|
Change at Week 52b |
-0.4 |
-0.4 |
+4.4 |
|
Difference from placebo + met + glimb (95% CI) |
-0.0 (-0.9, 0.8) | ||
|
Difference from pioglitazone + met + glimb (95% CI) |
-4.9 (-5.5, -4.2)c |
- a Intent-to-treat population. Last observation carried forward (LOCF) was used to impute missing data. Data post-onset of rescue therapy are treated as missing. At Week 52, primary efficacy data was imputed for 70%, 35%, and 34% of individuals randomized to placebo, TANZEUM, and pioglitazone, respectively.
- b Least squares mean adjusted for baseline value and stratification factors.
- c P <0.0001 for treatment difference.
- d Did not meet non-inferiority margin of 0.3%.
Combination Therapy: Active-Controlled Trial versus Liraglutide
The efficacy of TANZEUM was evaluated in a 32-week, randomized, open-label, liraglutide-controlled, non-inferiority trial in 805 patients with type 2 diabetes mellitus inadequately controlled on monotherapy or combination oral antidiabetic therapy (metformin, thiazolidinedione, sulfonylurea, or a combination of these). Patients were randomized to TANZEUM 30 mg SC weekly (with uptitration to 50 mg weekly at Week 6) or liraglutide 1.8 mg daily (titrated up from 0.6 mg at Week 1, and from 1.2 mg at Week 2). The mean age of participants was 56 years, 50% of patients were men, the mean duration of type 2 diabetes was 8 years, and the mean baseline eGFR was 95 mL/min/1.73 m2. Results of the primary and main secondary analyses are presented in Table 8.
The between-treatment difference of 0.2% with 95% confidence interval (0.08, 0.34) between TANZEUM and liraglutide did not meet the pre-specified, non-inferiority margin (0.3%). In this trial, TANZEUM provided less HbA1c reduction than liraglutide and the treatment difference was statistically significant (see Table 8).
Table 8. Results of Controlled Trial of TANZEUM versus Liraglutide at Week 32 (LOCFa)
|
TANZEUM |
Liraglutide |
|
|
ITTa (n) |
402 |
403 |
|
HbA1c (%) | ||
|
Baseline (mean) |
8.2% |
8.2% |
|
Change at Week 32b |
-0.8 |
-1.0 |
|
Difference from liraglutideb (95% CI) |
0.2 (0.08, 0.34)c | |
|
Proportion achieving HbA1c <7% |
42% |
52% |
|
FPG (mg/dL) | ||
|
Baseline (mean) |
169 |
167 |
|
Change at Week 32b |
-22 |
-30 |
|
Difference from liraglutideb (95% CI) |
8 (3, 14)d | |
|
Body Weight (kg) | ||
|
Baseline (mean) |
92 |
93 |
|
Change at Week 32b |
-0.6 |
-2.2 |
|
Difference from liraglutideb (95% CI) |
1.6 (1.1, 2.1)d |
- a Intent-to-treat population. Last observation carried forward (LOCF) was used to impute missing data. Data post-onset of rescue therapy are treated as missing. At Week 32, primary efficacy data was imputed for 31% and 24% of individuals randomized to TANZEUM and liraglutide, respectively.
- b Least squares mean adjusted for baseline value and stratification factors.
- c Did not meet non-inferiority margin of 0.3%.
- d P <0.005 for treatment difference in favor of liraglutide.
Combination Therapy: Active-Controlled Trial versus Basal Insulin
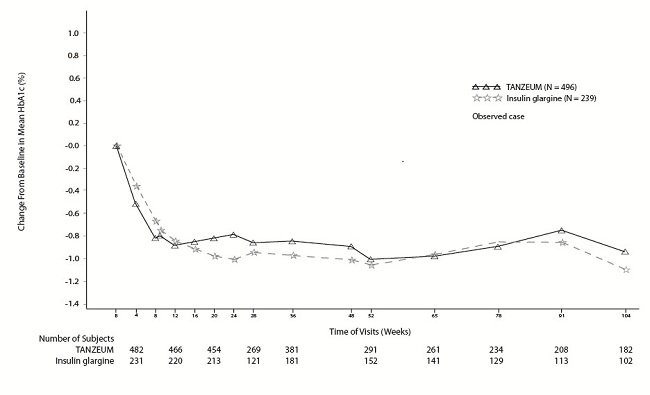
The efficacy of TANZEUM was evaluated in a 52-week, randomized (2:1), open-label, insulin glargine-controlled, non-inferiority trial in 735 patients with type 2 diabetes mellitus inadequately controlled on metformin ≥1,500 mg daily (with or without sulfonylurea). Patients were randomized to receive TANZEUM 30 mg SC weekly (with optional uptitration to 50 mg weekly) or insulin glargine (median starting dose of 10 units and titrated weekly per prescribing information). The primary endpoint was change in HbA1c from baseline compared with insulin glargine. The starting total daily dose of insulin glargine ranged between 2 and 40 units (median daily dose of 10 units) and ranged between 3 and 230 units (median daily dose of 30 units) at Week 52. Sixty-nine percent of patients treated with TANZEUM were uptitrated to 50 mg SC weekly. The mean age of participants was 56 years, 56% of patients were men, the mean duration of type 2 diabetes was 9 years, and the mean baseline eGFR was 85 mL/min/1.73 m2. Results of the primary and main secondary analyses are presented in Table 9.
The between-treatment difference of 0.1% with 95% confidence interval (-0.04%, 0.27%) for TANZEUM and insulin glargine met the pre-specified, non-inferiority margin (0.3%). A mean decrease in body weight was observed for TANZEUM compared with a mean increase in body weight for insulin glargine, and the difference in weight change was statistically significant (see Table 9).
Table 9. Results at Week 52 (LOCFa) in a Trial Comparing TANZEUM with Insulin Glargine as Add-On Therapy in Patients Inadequately Controlled on Metformin ± Sulfonylurea
|
TANZEUM + Metformin (with or without Sulfonylurea) |
Insulin Glargine + Metformin (with or without Sulfonylurea) |
|
|
ITTa (n) |
496 |
239 |
|
HbA1c (%) | ||
|
Baseline (mean) |
8.3 |
8.4 |
|
Change at Week 52b |
-0.7 |
-0.8 |
|
Difference from insulin glargineb (95% CI) |
0.1 (-0.04, 0.27)c | |
|
Proportion achieving HbA1c <7% |
32 |
33 |
|
FPG (mg/dL) | ||
|
Baseline (mean) |
169 |
175 |
|
Change at Week 52b |
-16 |
-37 |
|
Difference from insulin glargineb (95% CI) |
21 (14, 29)d | |
|
Body Weight (kg) | ||
|
Baseline (mean) |
95 |
95 |
|
Change at Week 52b |
-1.1 |
1.6 |
|
Difference from insulin glargineb (95% CI) |
-2.6 (-3.2, -2.0)e |
- a Intent-to-treat population. Last observation carried forward (LOCF) was used to impute missing data. Data post-onset of rescue therapy are treated as missing. At Week 52, primary efficacy data was imputed for 41% and 36% of individuals randomized to TANZEUM and insulin glargine, respectively.
- b Least squares mean adjusted for baseline value and stratification factors.
- c Met non-inferiority margin of 0.3%.
- d P <0.0001 in favor of insulin glargine.
- e P <0.0001.
Figure 3. Mean HbA1c Change from Baseline (Completers) in a Trial Comparing TANZEUM with Insulin Glargine as Add-On Therapy in Patients Inadequately Controlled on Metformin (with or without a Sulfonylurea)
Combination Therapy: Active-Controlled Trial versus Prandial Insulin
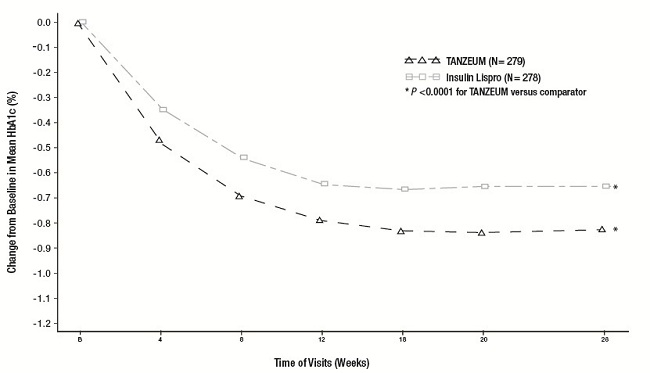
The efficacy of TANZEUM was evaluated in a 26-week, randomized, open-label, multicenter, non-inferiority trial in 563 patients with type 2 diabetes mellitus inadequately controlled on insulin glargine (≥20 units per day). Patients were randomized to receive TANZEUM 30 mg SC once weekly (with uptitration to 50 mg if inadequately controlled after Week 8) or insulin lispro (administered daily at meal times, started according to standard of care and titrated to effect). At Week 26, the mean daily dose of insulin glargine was 53 IU for TANZEUM and 51 IU for insulin lispro. The mean daily dose of insulin lispro at Week 26 was 31 IU, and 51% of patients treated with TANZEUM were on 50 mg weekly. The mean age of participants was 56 years, 47% of patients were men, the mean duration of type 2 diabetes was 11 years, and the mean baseline eGFR was 91 mL/min/1.73 m2. Results of the primary and main secondary analyses are presented in Table 10. Figure 4 shows the mean adjusted changes in HbA1c from baseline across study visits.
The between-treatment difference of -0.2% with 95% confidence interval (-0.32%, 0.00%) between albiglutide and insulin lispro met the pre-specified non-inferiority margin (0.4%). Treatment with TANZEUM resulted in a mean weight loss for TANZEUM compared with a mean weight gain for insulin lispro, and the difference between treatment groups was statistically significant (see Table 10).
Table 10. Results at Week 26 (LOCFa) in a Trial Comparing TANZEUM with Insulin Lispro as Add-On Therapy in Patients Inadequately Controlled on Insulin Glargine
|
TANZEUM + Insulin Glargine |
Insulin Lispro + Insulin Glargine |
|
|
ITTa (n) |
282 |
281 |
|
HbA1c (%) | ||
|
Baseline (mean) |
8.5 |
8.4 |
|
Change at Week 26b |
-0.8 |
-0.7 |
|
Difference from insulin lisprob (95% CI) |
-0.2(-0.32, 0.00)c | |
|
Proportion achieving HbA1c <7% |
30% |
25% |
|
FPG (mg/dL) | ||
|
Baseline (mean) |
153 |
153 |
|
Change at Week 26b |
-18 |
-13 |
|
Difference from insulin lisprob (95% CI) |
-5 (-13, 3) | |
|
Body Weight (kg) | ||
|
Baseline (mean) |
93 |
92 |
|
Change at Week 26b |
-0.7 |
+0.8 |
|
Difference from insulin lisprob (95% CI) |
-1.5 (-2.1, -1.0)d |
- a Intent-to-treat population. Last observation carried forward (LOCF) was used to impute missing data. Data post-onset of rescue therapy are treated as missing. At Week 26, primary efficacy data was imputed for 29% and 29% of individuals randomized to TANZEUM and insulin lispro, respectively.
- b Least squares mean adjusted for baseline value and stratification factors.
- c Rules out a non-inferiority margin of 0.4%.
- d P <0.0001 for treatment difference.
Figure 4. Mean HbA1c Change from Baseline (ITT-LOCF population) in a Trial Comparing TANZEUM with Insulin Lispro as Add-On Therapy in Patients Inadequately Controlled on Insulin Glargine
14.3 Type 2 Diabetes Mellitus Patients with Renal Impairment
The efficacy of TANZEUM was evaluated in a 26-week, randomized, double-blind, active-controlled trial in 486 patients with mild (n = 250), moderate (n = 200), and severe renal impairment (n = 36) inadequately controlled on a current regimen of diet and exercise or other antidiabetic therapy. Patients were randomized to receive TANZEUM 30 mg SC weekly (with uptitration to 50 mg weekly if needed as early as Week 4) or sitagliptin. Sitagliptin was dosed according to renal function (100 mg, 50 mg, and 25 mg daily in mild, moderate, and severe renal impairment, respectively). The mean age of participants was 63 years, 54% of patients were men, the mean duration of type 2 diabetes was 11 years, and the mean baseline eGFR was 60 mL/min/1.73 m2.
Results of the primary and main secondary analyses are presented in Table 11. Treatment with TANZEUM resulted in statistically significant reductions in HbA1c from baseline at Week 26 compared with sitagliptin (see Table 11).
Table 11. Results at Week 26 (LOCFa) in a Trial Comparing TANZEUM with Sitagliptin in Patients with Renal Impairment
|
TANZEUM |
Sitagliptin |
|
|
ITTa (n) |
246 |
240 |
|
HbA1c (%) | ||
|
Baseline (mean) |
8.1 |
8.2 |
|
Change at Week 26b |
-0.8 |
-0.5 |
|
Difference from sitagliptinb (95% CI) |
-0.3 (-0.49, -0.15)c | |
|
Proportion achieving HbA1c <7% |
43% |
31% |
|
FPG (mg/dL) | ||
|
Baseline (mean) |
166 |
165 |
|
Change at Week 26b |
-26 |
-4 |
|
Difference from sitagliptinb (95% CI) |
-22 (-31, -13)c | |
|
Body Weight (kg) | ||
|
Baseline (mean) |
84 |
83 |
|
Change at Week 26b |
-0.8 |
-0.2 |
|
Difference from sitagliptinb (95% CI) |
-0.6 (-1.1, -0.1)d |
- a Intent-to-treat population. Last observation carried forward (LOCF) was used to impute missing data. Data post-onset of rescue therapy are treated as missing. At Week 26 primary efficacy data was imputed for 17% and 25% of individuals randomized to TANZEUM and sitagliptin, respectively.
- b Least squares mean adjusted for baseline value and stratification factors.
- c P <0.0003 for treatment difference.
- d P = 0.0281 for treatment difference.
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
TANZEUM is available in the following strengths and package size:
30-mg single-dose Pen (NDC 0173-0866-01):
- •
- carton of 4 (containing four 29-gauge, 5-mm, thinwall needles): NDC 0173-0866-35
50-mg single-dose Pen (NDC 0173-0867-01):
- •
- carton of 4 (containing four 29-gauge, 5-mm, thinwall needles): NDC 0173-0867-35
16.2 Storage and Handling
- •
- Prior to dispensing: Store Pens in the refrigerator at 36°F to 46°F (2°C to 8°C). Pens may be stored refrigerated until the expiration date.
- •
- Following dispensing: Store Pens in the refrigerator at 36°F to 46°F (2°C to 8°C). Patients may store Pens at room temperature not to exceed 86°F (30°C) for up to 4 weeks prior to use. Store Pens in the original carton until use.
- •
- Do not freeze.
- •
- Do not use past the expiration date.
- •
- Use within 8 hours after reconstitution.
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use). The Medication Guide is contained in a separate leaflet that accompanies the product.
- •
- Instruct patients to read the Instructions for Use including the Frequently Asked Questions before starting therapy and to read again each time before injecting the dose. Instruct patients on proper use, storage, and disposal of the pen [see How Supplied/Storage and Handling (16.2), Patient Instructions for Use].
- •
- Inform patients about self-management practices, including the importance of proper storage of TANZEUM, injection technique, timing of dosage of TANZEUM and concomitant oral drugs, and recognition and management of hypoglycemia.
- •
- Inform patients that thyroid C-cell tumors have been observed in rodents treated with some GLP-1 receptor agonists, and the human relevance of this finding has not been determined. Counsel patients to report symptoms of thyroid tumors (e.g., a lump in the neck, dysphagia, dyspnea, or persistent hoarseness) to their physician [see Boxed Warning, Warnings and Precautions (5.1)].
- •
- Advise patients that persistent, severe abdominal pain that may radiate to the back and which may (or may not) be accompanied by vomiting is the hallmark symptom of acute pancreatitis. Instruct patients to discontinue TANZEUM promptly and to contact their physician if persistent, severe abdominal pain occurs [see Warnings and Precautions (5.2)].
- •
- The risk of hypoglycemia is increased when TANZEUM is used in combination with an agent that induces hypoglycemia, such as sulfonylurea or insulin. Instructions for hypoglycemia should be reviewed with patients and reinforced when initiating therapy with TANZEUM, particularly when concomitantly administered with a sulfonylurea or insulin [see Warnings and Precautions (5.3)].
- •
- Inform patients that serious hypersensitivity reactions have been reported with use of TANZEUM. Advise patients on the symptoms of hypersensitivity reactions and instruct them to stop taking TANZEUM and seek medical advice promptly if such symptoms occur [see Warnings and Precautions (5.4)].
- •
- Advise patients of the potential risk of dehydration in relation to gastrointestinal side effects and to take precautions to avoid fluid depletion [see Warnings and Precautions (5.5)].
- •
- Instruct patients to read the Medication Guide before starting TANZEUM and to read again each time the prescription is renewed. Instruct patients to inform their doctor or pharmacist if they develop any unusual symptom, or if any known symptom persists or worsens.
- •
- Inform patients not to take an extra dose of TANZEUM to make up for a missed dose. If a dose is missed, instruct patients to take a dose as soon as possible within 3 days after the missed dose. Instruct patients to then take their next dose at their usual weekly time. If it has been longer than 3 days after the missed dose, instruct patients to wait and take TANZEUM at the next usual weekly time.
Trademark is owned by or licensed to the GSK group of companies.
Manufactured by GlaxoSmithKline LLC
Wilmington, DE 19808
U.S. Lic. No. 1727
Marketed by GlaxoSmithKline
Research Triangle Park, NC 27709
©2017 GSK group of companies or its licensor.
TNZ:8PI
|
Medication Guide TANZEUM (TAN-zee-um) (albiglutide) for injection, for subcutaneous use |
|
Read this Medication Guide before you start using TANZEUM and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment. |
|
What is the most important information I should know about TANZEUM? TANZEUM may cause serious side effects, including:
|
|
What is TANZEUM? TANZEUM is an injectable prescription medicine that may improve blood sugar (glucose) in adults with type 2 diabetes mellitus, and should be used along with diet and exercise.
|
|
Who should not use TANZEUM? Do not use TANZEUM if:
|
|
What should I tell my healthcare provider before using TANZEUM? Before using TANZEUM, tell your healthcare provider if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. TANZEUM may affect the way some medicines work and some medicines may affect the way TANZEUM works. Before using TANZEUM, talk to your healthcare provider about low blood sugar and how to manage it. Tell your healthcare provider if you are taking other medicines to treat diabetes including insulin or sulfonylureas. Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine. |
|
How should I use TANZEUM?
Do not share your TANZEUM pen or needles with another person. You may give another person an infection or get an infection from them. Your dose of TANZEUM and other diabetes medicines may need to change because of: change in level of physical activity or exercise, weight gain or loss, increased stress, illness, change in diet, or because of other medicines you take. |
|
What are the possible side effects of TANZEUM? TANZEUM may cause serious side effects, including:
The most common side effects of TANZEUM may include:
Talk to your healthcare provider about any side effect that bothers you or does not go away. These are not all the possible side effects of TANZEUM. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|
General information about the safe and effective use of TANZEUM. Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use TANZEUM for a condition for which it was not prescribed. Do not give TANZEUM to other people, even if they have the same symptoms that you have. It may harm them. This Medication Guide summarizes the most important information about TANZEUM. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about TANZEUM that is written for health professionals. |
|
What are the ingredients in TANZEUM? Active Ingredient: albiglutide Inactive Ingredients: mannitol, polysorbate 80, sodium phosphate, and trehalose dihydrate. TANZEUM does not contain a preservative. |
|
Manufactured by GlaxoSmithKline LLC Wilmington, DE 19808 U.S. Lic No. 1727 Marketed by GlaxoSmithKline Research Triangle Park, NC 27709 Trademark is owned by or licensed to the GSK group of companies. ©2017 GSK group of companies or its licensor. TNZ:6MG For more information, go to www.TANZEUM.com or call 1-888-825-5249. |
This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: December 2017
INSTRUCTIONS FOR USE
TANZEUM® (TAN-zee-um)
(albiglutide)
for injection, for subcutaneous use
TANZEUM (albiglutide) Pen 30 mg
Before you Begin: Wash Your Hands, Gather and Inspect Your Supplies
- •
- Wash your hands.
- •
- Take a pen and new needle out of the box and check the label on your pen to make sure it is your prescribed dose of medicine.
- •
- Gather a clean, empty cup to hold the pen while the medicine mixes, a clock timer to measure the time while the medicine mixes, and a large sharps container for pen disposal. See “Disposing of Your Used Pens and Needles” at the end of these instructions.
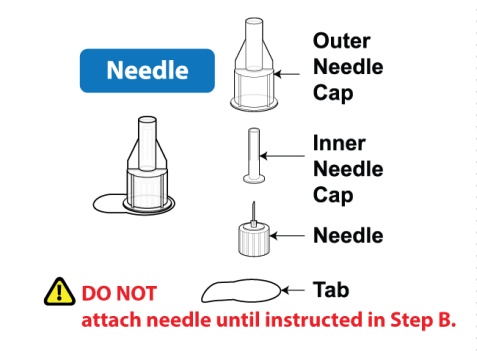 |  |
|
 |  |  |
Step A
Inspect Your Pen and Mix Your Medicine
Inspect Your Pen
- •
- Make sure that you have all of the supplies listed above (pen, needle, cup, timer, sharps container).
- •
- Check the expiration date on the pen. Do not use if expired.
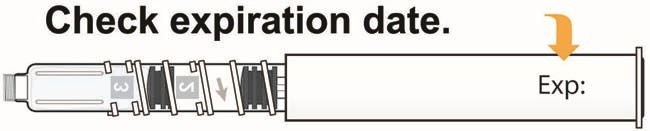 |
- •
- Check that the pen has a [1] in the number window.
Do not use if the [1] is not showing.
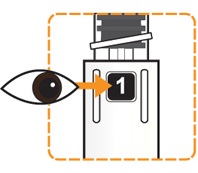 |
Twist Pen to Mix Your Medicine
- •
- Hold the pen body with the clear cartridge pointing up so that you see the [1] in the number window.
- •
- With your other hand, twist the clear cartridge several times in the direction of the arrow (clockwise) until you feel and hear the pen “click” into place and you see the [2] in the number window. This will mix the medicine powder and liquid in the clear cartridge.
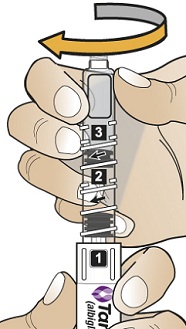 | 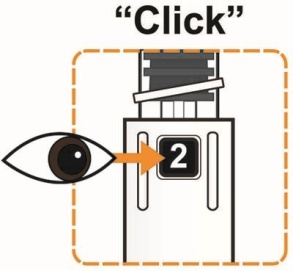 |
- •
- Slowly and gently rock the pen side to side (like a windshield wiper) 5 times to mix the medicine. Do not shake the pen hard to avoid foaming; it may affect your dose.
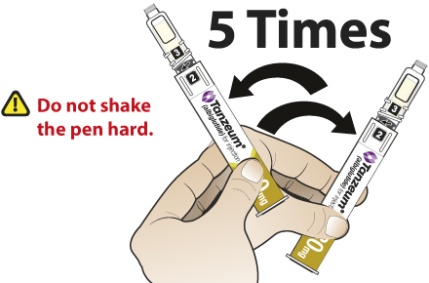 |
Wait for Medicine to Dissolve
- •
- Place the pen into the clean, empty cup to keep the clear cartridge pointing up.
- •
- Set the clock timer for 15 minutes.
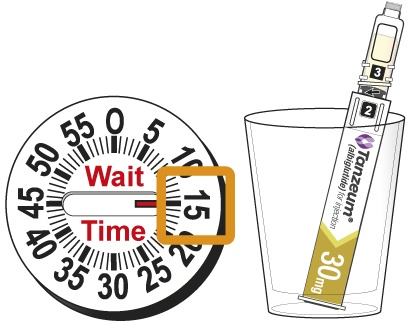 |
|
You must wait 15 minutes for the medicine to dissolve before continuing to Step B. |
Step B
Attach the Needle and Prepare the Pen for Injection
After the 15 minute wait, wash your hands and finish the rest of the steps right away.
Inspect Your Dissolved Medicine
- •
- Again, slowly and gently rock the pen side to side (like a windshield wiper) 5 times to mix the medicine again. Do not shake the pen hard to avoid foaming; it may affect your dose.
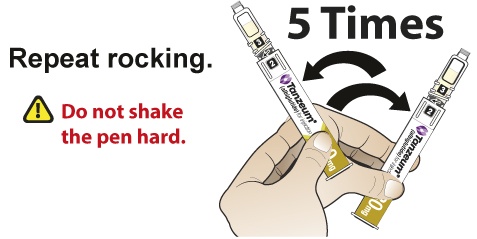 |
- •
- Look through the viewing window to check that the liquid in the cartridge is clear and free of solid particles.
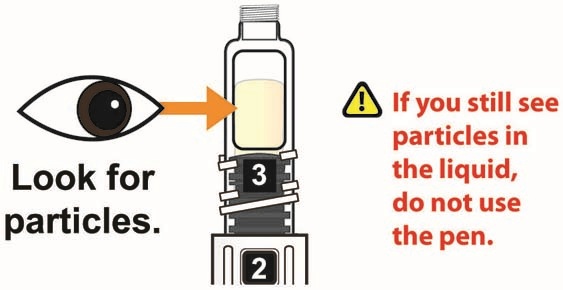 |
- •
- The liquid will have a yellow color and there will be large air bubbles on top of the liquid.
Attach the Needle
- •
- Peel the tab from the outer needle cap.
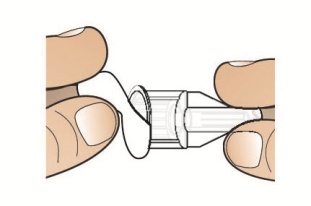 |
- •
- Hold the pen with the clear cartridge pointing up and push the needle straight down onto the clear cartridge until you hear a “click” and feel the needle “snap” down into place. This means the needle is attached.
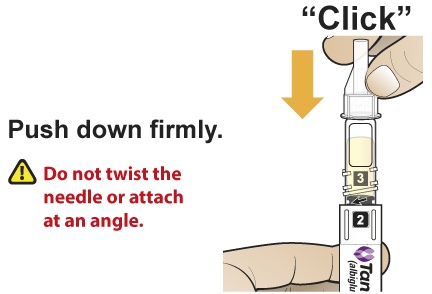 |
Tap for Air Bubbles
- •
- With the needle point up, gently tap the clear cartridge 2 to 3 times to bring large air bubbles to the top.
 |
|
Small bubbles are okay and do not need to rise to the top. |
Twist Pen to Prime the Needle
- •
- After the needle is attached, slowly twist the clear cartridge several times in the direction of the arrow (clockwise) until you feel and hear the pen “click” and you see the [3] in the number window. This removes the large air bubbles from the clear cartridge. The injection button will also pop out from the bottom of the pen.
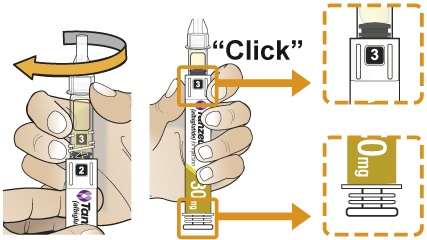 |
Step C
Remove Both Needle Caps and Inject Your Medicine
Remove Needle Caps
- •
- Carefully remove the outer needle cap, then the inner needle cap. A few drops of liquid may come out of the needle. This is normal.
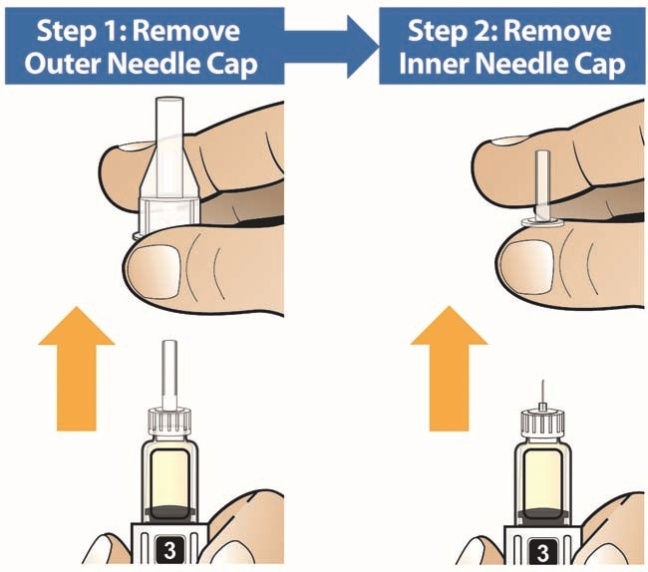 |
Inject the Medicine
- •
- Insert the needle into the skin on your abdomen, thigh, or upper arm and inject as shown to you by your healthcare provider.
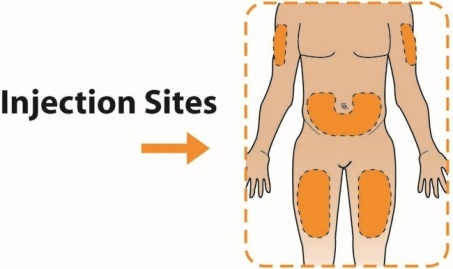 |
- •
- With your thumb, press the injection button slowly and steadily to inject your medicine. The slower you press the button, the less pressure you will feel.
- •
- Keep the injection button pressed down until you hear a “click”. After hearing the click, continue holding your thumb down on the button and then slowly count to 5 to deliver the full dose of the medicine.
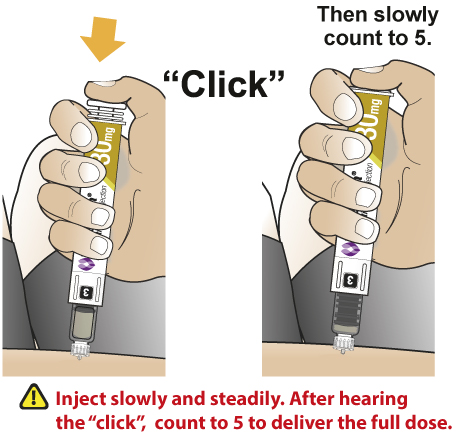 |
- •
- After hearing the “click” and then slowly counting to 5, pull the needle out of your skin.
Disposing of Your Used Pens and Needles
- •
- Do not recap the needle or remove needle from the pen.
- •
- Put your used needles and pens in an FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles and pens in your household trash.
 |
General Information About the Safe and Effective Use of TANZEUM
- •
- Take 1 time each week. You can take your medicine at any time of day, with or without meals.
- •
- Your healthcare provider will teach you how to mix and inject TANZEUM before you use it for the first time. If you have questions or do not understand the Instructions for Use, talk to your healthcare provider.
- •
- Use TANZEUM exactly as your healthcare provider tells you. Do not change your dose or stop TANZEUM without talking to your healthcare provider.
- •
- Change (rotate) your injection site with each injection (weekly).
- •
- TANZEUM is injected under the skin (subcutaneously) in your stomach area (abdomen), upper leg (thigh), or upper arm.
- •
- Do not inject TANZEUM into a vein or muscle.
- •
- If you use TANZEUM with insulin, you should inject your TANZEUM and insulin separately. Do not mix insulin and TANZEUM together. You can inject TANZEUM and insulin in the same body area (for example, your stomach area), but you should not give the injections right next to each other.
- •
- Keep pens and needles out of the reach of children.
- •
- Always use a new needle for each injection.
- •
- Do not share pens or needles.
Frequently Asked Questions
Medicine Dosing
What if I need to take my medicine on a different day of the week?
- •
- You may take your next dose of medicine on a different day as long as it has been at least 4 days since your last dose.
What if I forget to take the medicine on the day I am supposed to?
- •
- Take your missed dose of medicine within 3 days after your scheduled day, then return to your scheduled day for your next dose. If more than 3 days have passed since your usual scheduled day, wait until your next regularly scheduled day to take the injection of TANZEUM.
Storage
How should I store my medicine?
- •
- Store your pens in the refrigerator between 36°F to 46°F (2°C to 8°C).
- •
- You may store your pen in the box at room temperature below 86°F (30°C) for up to 4 weeks before you are ready to use the pen.
- •
- Store pens in the carton they came in.
- •
- Do not freeze pens. If the liquid in the pen is frozen, throw away the pen and use another pen.
Number Window
Are the Numbers 1, 2, and 3 used to select my dose of medicine?
- •
- No, you do not have to select your dose. The numbers are to help you prepare and give your medicine.
Number 1: Pen is ready to begin. Medicine powder and water are in separate compartments in the clear cartridge. If you don’t see a number 1 in the window, throw away the pen.
Number 2: Medicine powder and water are mixed and then gently rocked. Wait 15 minutes, then attach needle.
Number 3: Large air bubbles are removed, the injection button pops out, and the pen is ready for injection.
Step A: Inspect Your Pen and Mix Your Medicine
What if I do not wait 15 minutes after turning the pen to the Number 2?
- •
- If you do not wait the full 15 minutes the medicine may not be mixed with the water the right way. This can result in particles floating in the clear cartridge, not getting your full dose, or a blocked needle. Waiting the full 15 minutes ensures that the medicine powder and water are mixed the right way, even though it may look like it is mixed sooner.
What if I leave my pen for more than 15 minutes after turning the pen to the Number 2 in Step A?
- •
- As long as the needle has not been attached, the pen can be used for up to 8 hours from the time Step A was started. If it has been more than 8 hours since the medicine was mixed in Step A, throw away the pen and use another pen.
- •
- If you have attached the needle, TANZEUM should be used right away.
Step B: Attach the Needle and Prepare Pen for Injection
What if I leave my pen with the needle attached at Step B, and come back later to finish Step C?
- •
- This can cause your needle to block, you should continue from Step B to Step C right away.
What if I do not attach the needle at Step B as instructed?
- •
- If the needle is attached at Step A, some of the medicine may be lost during mixing. Do not attach the needle at Step A.
- •
- Attaching the needle while the number 2 is in the window allows the air inside the cartridge to escape through the needle. If you do not click the needle on or if you start turning the cartridge before attaching the needle, the pen may not deliver the full dose.
- •
- If the pen is jammed or leaking, throw it away and use another pen.
What if I do not hear the “click” when the 2 or when the 3 is moved into the Number Window?
- •
- If you do not hear a “click” when the 2 or when the 3 is moved into the number window, you may not have the number fully centered in the window. Twist the clear cartridge slightly in the direction of the arrows (clockwise) to complete the “click” and center the number in the window.
- •
- If you are unable to turn to position 3, throw it away and use another pen.
Step C: Remove Both Needle Caps and Inject Your Medicine
After I turn the pen to Number 3 (Step B), there are still some small air bubbles remaining. Can I still use the pen?
- •
- Seeing small air bubbles remaining is normal and you can still use the pen.
After I give my medicine, there is some liquid still seen in the clear cartridge.
- •
- This is normal. If you have heard and felt the injection button “click” and slowly counted to 5 before pulling the needle out of your skin, you should have received the full dose of your medicine.
How should I dispose of the pen?
- •
- Do not recap the needle or remove needle from the pen.
- •
- Put your used needles and pens in an FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles and pens in your household trash.
- •
- If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- o
- made of a heavy-duty plastic,
- o
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- o
- upright and stable during use,
- o
- leak-resistant, and
- o
- properly labeled to warn of hazardous waste inside the container.
- •
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and pens. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal.
- •
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
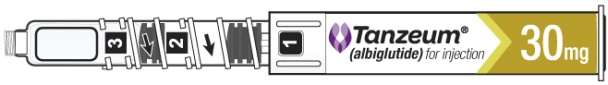 |
Please make sure you are using the right dose. These instructions are for the 30 mg dose. |
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Revised: September 2016
|
Manufactured by GlaxoSmithKline LLC Wilmington, DE 19808 U.S. Lic No. 1727 Marketed by GlaxoSmithKline Research Triangle Park, NC 27709 |
TANZEUM is a registered trademark of the GSK group of companies. ©2016 the GSK group of companies. All rights reserved. TNZ:4IFU-30 |
INSTRUCTIONS FOR USE
TANZEUM® (TAN-zee-um)
(albiglutide)
for injection, for subcutaneous use
TANZEUM (albiglutide) Pen 50 mg
Before you Begin: Wash Your Hands, Gather and Inspect Your Supplies
- •
- Wash your hands.
- •
- Take a pen and new needle out of the box and check the label on your pen to make sure it is your prescribed dose of medicine.
- •
- Gather a clean, empty cup to hold the pen while the medicine mixes, a clock timer to measure the time while the medicine mixes, and a large sharps container for pen disposal. See “Disposing of Your Used Pens and Needles” at the end of these instructions.
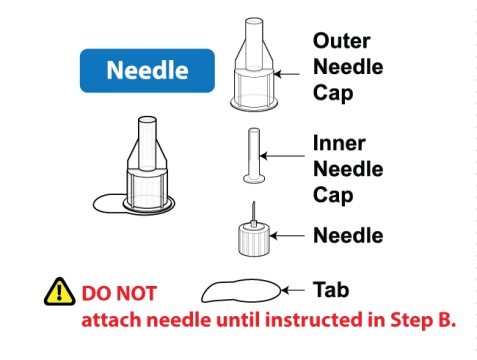 |  This TANZEUM 50 mg pen needs 30 minutes to let the medicine powder and water mix in Step A. This is different from the TANZEUM 30 mg pen you may have used before. |
 |  |  |
Step A
Inspect Your Pen and Mix Your Medicine
Inspect Your Pen
- •
- Make sure that you have all of the supplies listed above (pen, needle, cup, timer, sharps container).
- •
- Check the expiration date on the pen. Do not use if expired.
 |
- •
- Check that the pen has a [1] in the number window.
Do not use if the [1] is not showing.
 |
Twist Pen to Mix Your Medicine
- •
- Hold the pen body with the clear cartridge pointing up so that you see the [1] in the number window.
- •
- With your other hand, twist the clear cartridge several times in the direction of the arrow (clockwise) until you feel and hear the pen “click” into place and you see the [2] in the number window. This will mix the medicine powder and liquid in the clear cartridge.
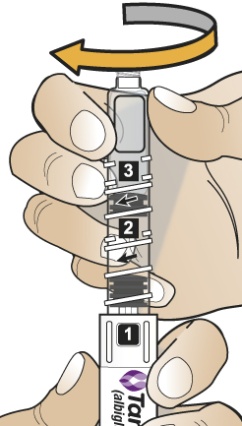 | 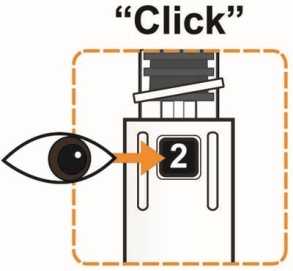 |
- •
- Slowly and gently rock the pen side to side (like a windshield wiper) 5 times to mix the medicine. Do not shake the pen hard to avoid foaming; it may affect your dose.
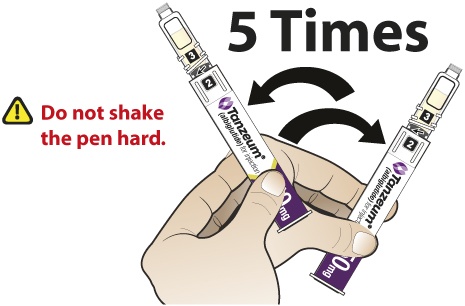 |
Wait for Medicine to Dissolve
- •
- Place the pen into the clean, empty cup to keep the clear cartridge pointing up.
- •
- Set the clock timer for 30 minutes.
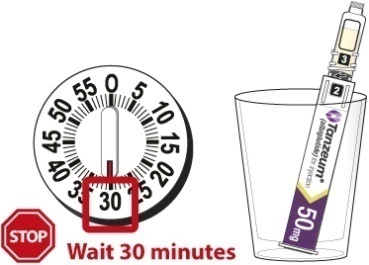 |
|
You must wait 30 minutes for the medicine to dissolve before continuing to Step B. |
Step B
Attach the Needle and Prepare the Pen for Injection
After the 30 minute wait, wash your hands and finish the rest of the steps right away.
Inspect Your Dissolved Medicine
- •
- Again, slowly and gently rock the pen side to side (like a windshield wiper) 5 times to mix the medicine again. Do not shake the pen hard to avoid foaming; it may affect your dose.
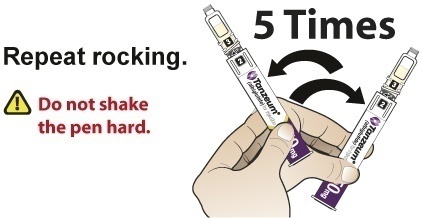 |
- •
- Look through the viewing window to check that the liquid in the cartridge is clear and free of solid particles.
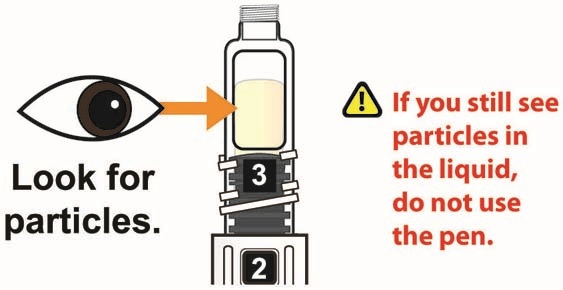 |
- •
- The liquid will have a yellow color and there will be large air bubbles on top of the liquid.
Attach the Needle
- •
- Peel the tab from the outer needle cap.
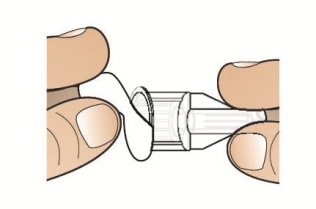 |
- •
- Hold the pen with the clear cartridge pointing up and push the needle straight down onto the clear cartridge until you hear a “click” and feel the needle “snap” down into place. This means the needle is attached.
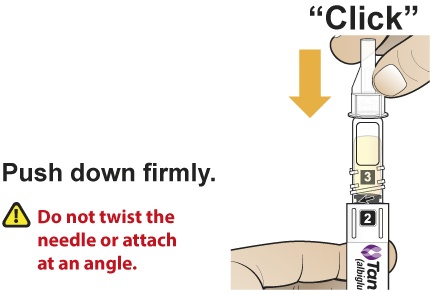 |
Tap for Air Bubbles
- •
- With the needle point up, gently tap the clear cartridge 2 to 3 times to bring large air bubbles to the top.
 |
|
Small bubbles are okay and do not need to rise to the top. |
Twist Pen to Prime the Needle
- •
- After the needle is attached, slowly twist the clear cartridge several times in the direction of the arrow (clockwise) until you feel and hear the pen “click” and you see the [3] in the number window. This removes the large air bubbles from the clear cartridge. The injection button will also pop out from the bottom of the pen.
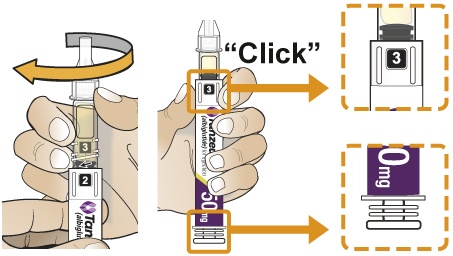 |
Step C
Remove Both Needle Caps and Inject Your Medicine
Remove Needle Caps
- •
- Carefully remove the outer needle cap, then the inner needle cap. A few drops of liquid may come out of the needle. This is normal.
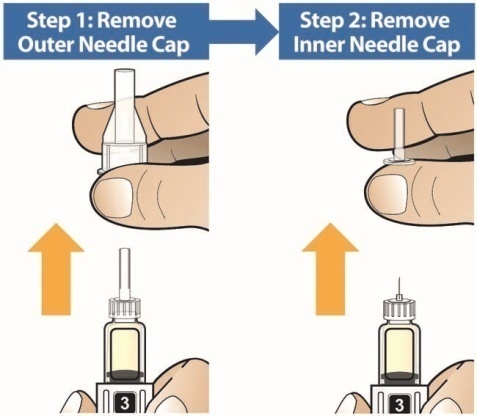 |
Inject the Medicine
- •
- Insert the needle into the skin on your abdomen, thigh, or upper arm and inject as shown to you by your healthcare provider.
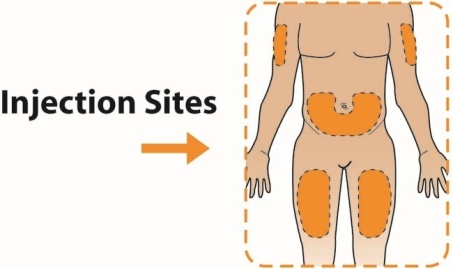 |
- •
- With your thumb, press the injection button slowly and steadily to inject your medicine. The slower you press the button, the less pressure you will feel.
- •
- Keep the injection button pressed down until you hear a “click”. After hearing the click, continue holding your thumb down on the button and then slowly count to 5 to deliver the full dose of the medicine.
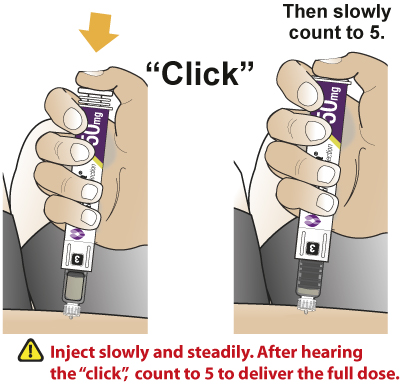 |
- •
- After hearing the “click” and then slowly counting to 5, pull the needle out of your skin.
Disposing of Your Used Pens and Needles
- •
- Do not recap the needle or remove needle from the pen.
- •
- Put your used needles and pens in an FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles and pens in your household trash.
 |
General Information About the Safe and Effective Use of TANZEUM
- •
- Take 1 time each week. You can take your medicine at any time of day, with or without meals.
- •
- Your healthcare provider will teach you how to mix and inject TANZEUM before you use it for the first time. If you have questions or do not understand the Instructions for Use, talk to your healthcare provider.
- •
- Use TANZEUM exactly as your healthcare provider tells you. Do not change your dose or stop TANZEUM without talking to your healthcare provider.
- •
- Change (rotate) your injection site with each injection (weekly).
- •
- TANZEUM is injected under the skin (subcutaneously) in your stomach area (abdomen), upper leg (thigh), or upper arm.
- •
- Do not inject TANZEUM into a vein or muscle.
- •
- If you use TANZEUM with insulin, you should inject your TANZEUM and insulin separately. Do not mix insulin and TANZEUM together. You can inject TANZEUM and insulin in the same body area (for example, your stomach area), but you should not give the injections right next to each other.
- •
- Keep pens and needles out of the reach of children.
- •
- Always use a new needle for each injection.
- •
- Do not share pens or needles.
Frequently Asked Questions
Medicine Dosing
What if I need to take my medicine on a different day of the week?
- •
- You may take your next dose of medicine on a different day as long as it has been at least 4 days since your last dose.
What if I forget to take the medicine on the day I am supposed to?
- •
- Take your missed dose of medicine within 3 days after your scheduled day, then return to your scheduled day for your next dose. If more than 3 days have passed since your usual scheduled day, wait until your next regularly scheduled day to take the injection of TANZEUM.
Storage
How should I store my medicine?
- •
- Store your pens in the refrigerator between 36°F to 46°F (2°C to 8°C).
- •
- You may store your pen in the box at room temperature below 86°F (30°C) for up to 4 weeks before you are ready to use the pen.
- •
- Store pens in the carton they came in.
- •
- Do not freeze pens. If the liquid in the pen is frozen, throw away the pen and use another pen.
Number Window
Are the Numbers 1, 2, and 3 used to select my dose of medicine?
- •
- No, you do not have to select your dose. The numbers are to help you prepare and give your medicine.
Number 1: Pen is ready to begin. Medicine powder and water are in separate compartments in the clear cartridge. If you don’t see a number 1 in the window, throw away the pen.
Number 2: Medicine powder and water are mixed and then gently rocked. Wait 30 minutes, then attach needle.
Number 3: Large air bubbles are removed, the injection button pops out, and the pen is ready for injection.
Step A: Inspect Your Pen and Mix Your Medicine
What if I do not wait 30 minutes after turning the pen to the Number 2?
- •
- If you do not wait the full 30 minutes the medicine may not be mixed with the water the right way. This can result in particles floating in the clear cartridge, not getting your full dose, or a blocked needle. Waiting the full 30 minutes ensures that the medicine powder and water are mixed the right way, even though it may look like it is mixed sooner.
What if I leave my pen for more than 30 minutes after turning the pen to the Number 2 in Step A?
- •
- As long as the needle has not been attached, the pen can be used for up to 8 hours from the time Step A was started. If it has been more than 8 hours since the medicine was mixed in Step A, throw away the pen and use another pen.
- •
- If you have attached the needle, TANZEUM should be used right away.
Step B: Attach the Needle and Prepare Pen for Injection
What if I leave my pen with the needle attached at Step B, and come back later to finish Step C?
- •
- This can cause your needle to block, you should continue from Step B to Step C right away.
What if I do not attach the needle at Step B as instructed?
- •
- If the needle is attached at Step A, some of the medicine may be lost during mixing. Do not attach the needle at Step A.
- •
- Attaching the needle while the number 2 is in the window allows the air inside the cartridge to escape through the needle. If you do not click the needle on or if you start turning the cartridge before attaching the needle, the pen may not deliver the full dose.
- •
- If the pen is jammed or leaking, throw it away and use another pen.
What if I do not hear the “click” when the 2 or when the 3 is moved into the Number Window?
- •
- If you do not hear a “click” when the 2 or when the 3 is moved into the number window, you may not have the number fully centered in the window. Twist the clear cartridge slightly in the direction of the arrows (clockwise) to complete the “click” and center the number in the window.
- •
- If you are unable to turn to position 3, throw it away and use another pen.
Step C: Remove Both Needle Caps and Inject Your Medicine
After I turn the pen to Number 3 (Step B), there are still some small air bubbles remaining. Can I still use the pen?
- •
- Seeing small air bubbles remaining is normal and you can still use the pen.
After I give my medicine, there is some liquid still seen in the clear cartridge.
- •
- This is normal. If you have heard and felt the injection button “click” and slowly counted to 5 before pulling the needle out of your skin, you should have received the full dose of your medicine.
How should I dispose of the pen?
- •
- Do not recap the needle or remove needle from the pen.
- •
- Put your used needles and pens in an FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles and pens in your household trash.
- •
- If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- o
- made of a heavy-duty plastic,
- o
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- o
- upright and stable during use,
- o
- leak-resistant, and
- o
- properly labeled to warn of hazardous waste inside the container.
- •
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and pens. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal.
- •
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
 |
Please make sure you are using the right dose. These instructions are for the 50 mg dose. |
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Revised: September 2016
|
Manufactured by GlaxoSmithKline LLC Wilmington, DE 19808 U.S. Lic No. 1727 Marketed by GlaxoSmithKline Research Triangle Park, NC 27709 |
TANZEUM is a registered trademark of the GSK group of companies. ©2016 the GSK group of companies. All rights reserved. TNZ:4IFU-50 |
PRINCIPAL DISPLAY PANEL
NDC 0173-0866-35
Tanzeum®
(albiglutide) for injection
Rx Only
Once-weekly
30mg
Each prefilled pen will deliver one 30-mg dose in 0.5 mL.
For subcutaneous use only.
Contents:
- •
- 4 SINGLE-DOSE PREFILLED PENS
- •
- 4 NEEDLES
- •
- PRODUCT LITERATURE (Read carefully the Instructions for Use)
Dispense the enclosed Medication Guide to each patient.
To fully dissolve the medicine the 30-mg pen must sit upright in a cup for 15 minutes.
©2016 the GSK group of companies
Made in Germany
Rev. 11/16
10004929-03
PRINCIPAL DISPLAY PANEL
NDC 0173-0867-35
Tanzeum®
(albiglutide) for injection
Rx Only
Once-weekly
50mg
Each prefilled pen will deliver one 50-mg dose in 0.5 mL.
For subcutaneous use only.
Contents:
- •
- 4 SINGLE-DOSE PREFILLED PENS
- •
- 4 NEEDLES
- •
- PRODUCT LITERATURE (Read carefully the Instructions for Use)
Dispense the enclosed Medication Guide to each patient.
To fully dissolve the medicine the 50-mg pen must sit upright in a cup for 30 minutes.
THE 50-MG PEN HAS A DIFFERENT WAIT TIME
IMPORTANT
TO FULLY DISSOLVE THE MEDICINE THE MUST SIT UPRIGHT IN A CUP FOR 30 MINUTES
©2016 the GSK group of companies
Made in Germany
Rev. 11/16
10004925-03
| TANZEUM
albiglutide injection, powder, lyophilized, for solution |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| TANZEUM
albiglutide injection, powder, lyophilized, for solution |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - GlaxoSmithKline LLC (167380711) |