PIROXICAM - piroxicam capsule
Zydus Lifesciences Limited
----------
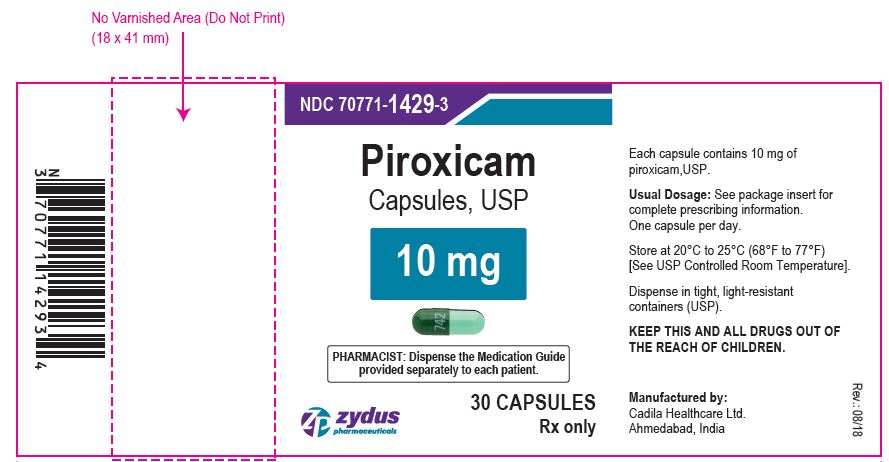
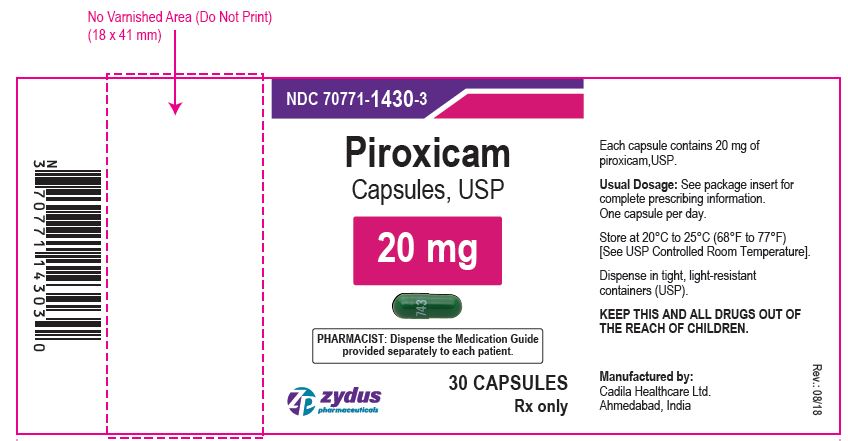
PIROXICAM CAPSULES
| PIROXICAM
piroxicam capsule |
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
| PIROXICAM
piroxicam capsule |
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Zydus Lifesciences Limited (918596198) |
Revised: 11/2022
Document Id: 20672ba1-c8fb-44a7-9e2b-1068b4503416
Set id: 5e43b2f2-f3b7-4c07-b1c8-b04f4739d3d2
Version: 4
Effective Time: 20221105
Zydus Lifesciences Limited