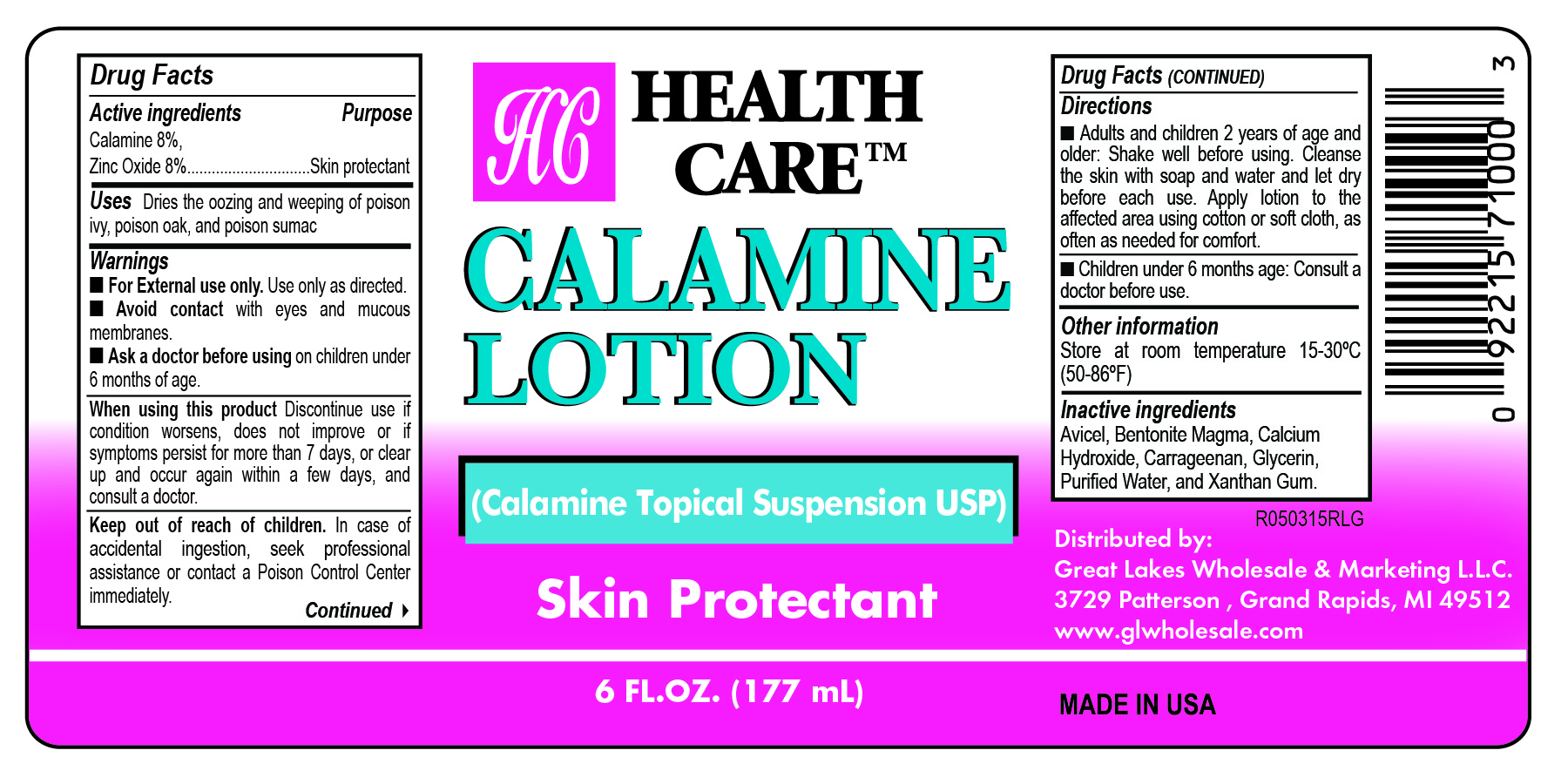
HEALTH CARE CALAMINE- calamine 8% and zinc oxide 8% lotion
Great Lakes Wholesale, Marketing and Sales Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Health Care Calamine Lotion
Uses
Dries the oozing and weeping of poison ivy, poison oak, and poison sumac and other skin irritations.
Ask a doctor
before using on children under 6 years of age.
Stop use and ask a doctor if
- Condition worsens
- Symptoms last for more than 7 days or clear up and occur again within a few days.
Keep out of reach of children.
In case of accidental ingestion, seek professional assistance or contact a Poison Control Center immediately.
| HEALTH CARE CALAMINE
calamine 8% and zinc oxide 8% lotion |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Great Lakes Wholesale, Marketing and Sales Inc (361925498) |
| Registrant - Pharma Nobis, LLC (118564114) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pharma Nobis, LLC | 118564114 | manufacture(64092-413) , analysis(64092-413) , pack(64092-413) , label(64092-413) | |
Revised: 3/2022
Document Id: da6aa5d8-d908-6ea1-e053-2995a90a3efa
Set id: 5d8f02fb-0794-8ec2-e053-2991aa0aac63
Version: 4
Effective Time: 20220317
Great Lakes Wholesale, Marketing and Sales Inc