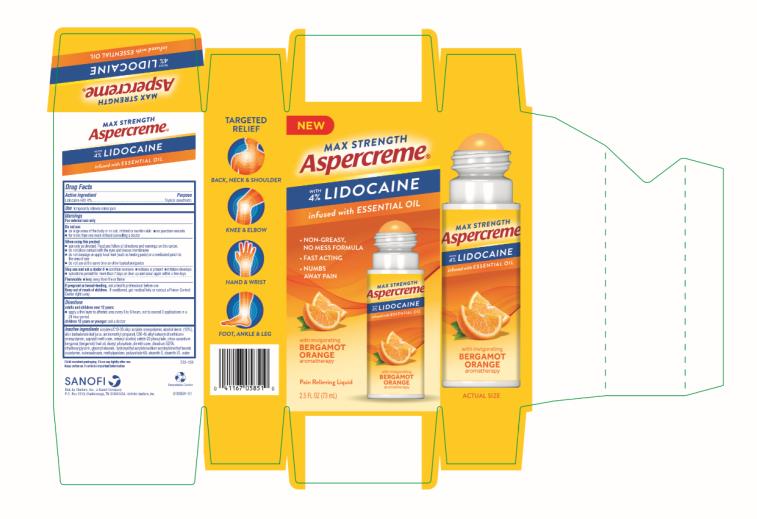
ASPERCREME LIDOCAINE NO-MESS BERGAMOT ORANGE- lidocaine hydrochloride liquid
Chattem, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Aspercreme with Lidocaine No-Mess Bergamot Orange
Warnings
For external use only
Do not use
■ on large areas of the body or on cut, irritated or swollen skin
■ on puncture wounds
■ for more than one week without consulting a doctor
When using this product
■ use only as directed. Read and follow all directions and warnings on this carton.
■ do not allow contact with the eyes and mucous membranes
■ do not bandage or apply local heat (such as heating pads) or a medicated patch to the area of use
■ do not use at the same time as other topical analgesics
Directions
adults and children over 12 years:
■ apply a thin layer to affected area every 6 to 8 hours, not to exceed 3 applications in a 24 hour period
children 12 years or younger: ask a doctor
Inactive ingredients
acrylates/C10-30 alkyl acrylate crosspolymer, alcohol denat. (15%), aloe barbadensis leaf juice, aminomethyl propanol, C30-45 alkyl cetearyl dimethicone crosspolymer, caprylyl methicone, cetearyl alcohol, ceteth-20 phosphate, citrus aurantium bergamia (bergamot) fruit oil, dicetyl phosphate, dimethicone, disodium EDTA, ethylhexylglycerin, glyceryl stearate, hydroxyethyl acrylate/sodium acryloyldimethyl taurate copolymer, isohexadecane, methylparaben, polysorbate 60, steareth-2, steareth-21, water
Child-resistant packaging. Close cap tightly after use.
Keep carton as it contains important information.
| ASPERCREME LIDOCAINE NO-MESS BERGAMOT ORANGE
lidocaine hydrochloride liquid |
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Chattem, Inc. (003336013) |