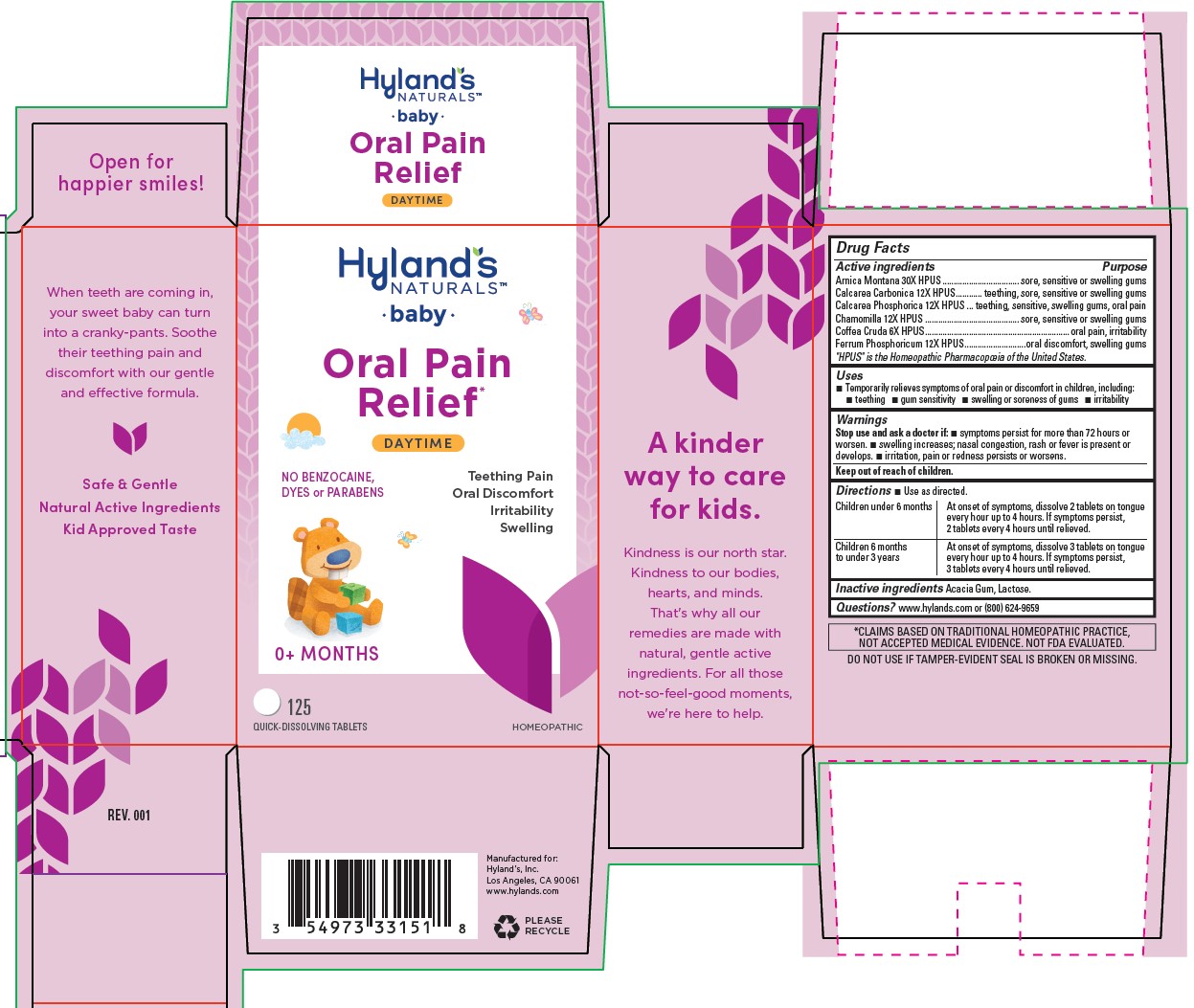
BABY ORAL PAIN RELIEF- oyster shell calcium carbonate, crude, tribasic calcium phosphate, arabica coffee bean, arnica montana, ferrum phosphoricum and matricaria chamomilla tablet
Hyland' Inc.
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Hyland's Naturals Baby Oral Pain Relief Daytime
Drug Facts
|
Active ingredients |
Purpose |
|
Arnica Montana 30X HPUS |
sore, sensitive or swelling gums |
|
Calcarea Carbonica 12X HPUS | sore, sensitive or swelling gums |
|
Calcarea Phosphorica 12X HPUS |
sensitive, swelling gums, oral pain |
|
Chamomilla 12X HPUS |
sore, sensitive or swelling gums |
|
Coffea Cruda 6X HPUS |
oral pain, irritability |
|
Ferrum Phosphoricum 12X HPUS |
oral discomfort, swelling gums |
“HPUS” indicates that the active ingredients are in the official Homeopathic Pharmacopœia of the United States.
Uses
■Temporarily relieves symptoms of oral pain or discomfort in children, including:
■ teething ■ gum sensitivity ■ swelling or soreness of gums ■ irritability
Warnings
Directions
■ Use as directed.
| Children under 6 months | At onset of symptoms, dissolve 2 tablets on tongue
every hour up to 4 hours. If symptoms persist, 2 tablets every 4 hours until relieved. |
| Children 6 months
to under 3 years | At onset of symptoms, dissolve 3 tablets on tongue
every hour up to 4 hours. If symptoms persist, 3 tablets every 4 hours until relieved. |
| BABY ORAL PAIN RELIEF
oyster shell calcium carbonate, crude, tribasic calcium phosphate, arabica coffee bean, arnica montana, ferrum phosphoricum and matricaria chamomilla tablet |
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| Labeler - Hyland' Inc. (008316655) |