Label: TOBRAMYCIN solution
- NDC Code(s): 66267-936-05
- Packager: NuCare Pharmaceuticals,Inc.
- This is a repackaged label.
- Source NDC Code(s): 24208-290
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated July 12, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Tobramycin Ophthalmic Solution, USP 0.3% is a sterile topical ophthalmic antibiotic formulation prepared specifically for topical therapy of external ophthalmic infections.
Each mL contains
Active: Tobramycin 3 mg (0.3%). Inactives: Boric Acid, Sodium Sulfate, Sodium Chloride, Tyloxapol and Purified Water. Sodium Hydroxide and/or Sulfuric Acid (to adjust pH). Tobramycin Ophthalmic Solution, USP 0.3% has a pH range between 7.0 and 8.0.
Preservative Added: Benzalkonium Chloride 0.1 mg (0.01%).
Tobramycin is a water-soluble aminoglycoside antibiotic active against a wide variety of gram-negative and gram-positive ophthalmic pathogens.
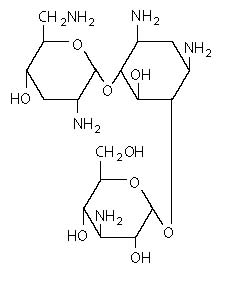
The chemical structure of tobramycin is
Molecular weight: 467.52
Molecular formula: C 18H 37N 5O 9
Chemical name:
(2S,3R,4S,5S,6R)-4-amino-2-[(1S,2S,3R,4S,6R)-4,6-diamino-3-[(2R,3R,5S,6R)-3-amino-6-(aminomethyl)-5-hydroxyoxan-2-yl]oxy-2-hydroxycyclohexyl]oxy-6-(hydroxymethyl)oxane-3,5-diol;sulfuric acid
-
CLINICAL PHARMACOLOGY
In Vitro Data: In vitro studies have demonstrated tobramycin is active against susceptible strains of the following microorganisms: Staphylococci, including S. aureus and S. epidermidis (coagulase-positive and coagulase-negative), including penicillin-resistant strains.
Streptococci, including some of the Group A-beta-hemolytic species, some nonhemolytic species, and some Streptococcus pneumoniae.
Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, Enterobacter aerogenes, Proteus mirabilis, Morganella morganii, most Proteus vulgaris strains, Haemophilus influenzae and H. aegyptius, Moraxella lacunata, Acinetobacter calcoaceticus and some Neisseria species. Bacterial susceptibility studies demonstrate that in some cases, microorganisms resistant to gentamicin retain susceptibility to tobramycin.
-
INDICATIONS AND USAGE
Tobramycin Ophthalmic Solution, USP 0.3% is a topical antibiotic indicated in the treatment of external infections of the eye and its adnexa caused by susceptible bacteria. Appropriate monitoring of bacterial response to topical antibiotic therapy should accompany the use of Tobramycin Ophthalmic Solution, USP 0.3%. Clinical studies have shown tobramycin to be safe and effective for use in children.
- CONTRAINDICATIONS
- WARNINGS
-
PRECAUTIONS
General
As with other antibiotic preparations, prolonged use may result in overgrowth of nonsusceptible organisms, including fungi. If superinfection occurs, appropriate therapy should be initiated. Cross-sensitivity to other aminoglycoside antibiotics may occur; if hypersensitivity develops with this product, discontinue use and institute appropriate therapy. Patients should be advised not to wear contact lenses if they have signs and symptoms of bacterial conjunctivitis.
Information For Patients
Do not touch dropper tip to any surface, as this may contaminate the solution.
Pregnancy Category B
Reproduction studies in three types of animals at doses up to thirty-three times the normal human systemic dose have revealed no evidence of impaired fertility or harm to the fetus due to tobramycin. There are, however, no adequate and well-controlled studies in pregnant women. Because animal studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers
Because of the potential for adverse reactions in nursing infants from Tobramycin Ophthalmic Solution, USP 0.3%, a decision should be made whether to discontinue nursing the infant or discontinue the drug, taking into account the importance of the drug to the mother.
-
ADVERSE REACTIONS
The most frequent adverse reactions to Tobramycin Ophthalmic Solution are hypersensitivity and localized ocular toxicity, including lid itching and swelling, and conjunctival erythema. These reactions occur in less than three of 100 patients treated with tobramycin. Similar reactions may occur with the topical use of other aminoglycoside antibiotics. Other adverse reactions have not been reported from tobramycin therapy; however, if topical ocular tobramycin is administered concomitantly with systemic aminoglycoside antibiotics, care should be taken to monitor the total serum concentration.
To report SUSPECTED ADVERSE REACTIONS, contact Bausch + Lomb, a division of Valeant Pharmaceuticals North America LLC, at 1-800-321-4576 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
- OVERDOSAGE
-
DOSAGE AND ADMINISTRATION
In mild to moderate disease, instill one or two drops into the affected eye(s) every four hours. In severe infections, instill two drops into the eye(s) hourly until improvement, following which treatment should be reduced prior to discontinuation.
DO NOT USE IF IMPRINTED NECKBAND IS NOT INTACT.
FOR TOPICAL OPHTHALMIC USE ONLY
-
HOW SUPPLIED
Tobramycin Ophthalmic Solution, USP 0.3% is supplied in a plastic bottle with a controlled drop tip and a white polypropylene cap in the following size:
NDC 66267-936-05 Box of 5mL
Storage
Store at 2°-25°C (36°-77°F). Avoid excessive heat.
KEEP OUT OF REACH OF CHILDREN.
Revised June 2017
Bausch + Lomb, a division of Valeant Pharmaceuticals North America LLC, Bridgewater, NJ 08807 USA
©Bausch & Lomb Incorporated
9116805 (folded)
9116905 (flat)
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
TOBRAMYCIN
tobramycin solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:66267-936(NDC:24208-290) Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TOBRAMYCIN (UNII: VZ8RRZ51VK) (TOBRAMYCIN - UNII:VZ8RRZ51VK) TOBRAMYCIN 3 mg in 1 mL Inactive Ingredients Ingredient Name Strength BORIC ACID (UNII: R57ZHV85D4) WATER (UNII: 059QF0KO0R) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM HYDROXIDE (UNII: 55X04QC32I) SODIUM SULFATE (UNII: 0YPR65R21J) SULFURIC ACID (UNII: O40UQP6WCF) TYLOXAPOL (UNII: Y27PUL9H56) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66267-936-05 5 mL in 1 BOX; Type 0: Not a Combination Product 09/08/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA064052 11/29/1993 Labeler - NuCare Pharmaceuticals,Inc. (010632300) Establishment Name Address ID/FEI Business Operations NuCare Phaarmaceuticals,Inc. 010632300 relabel(66267-936)