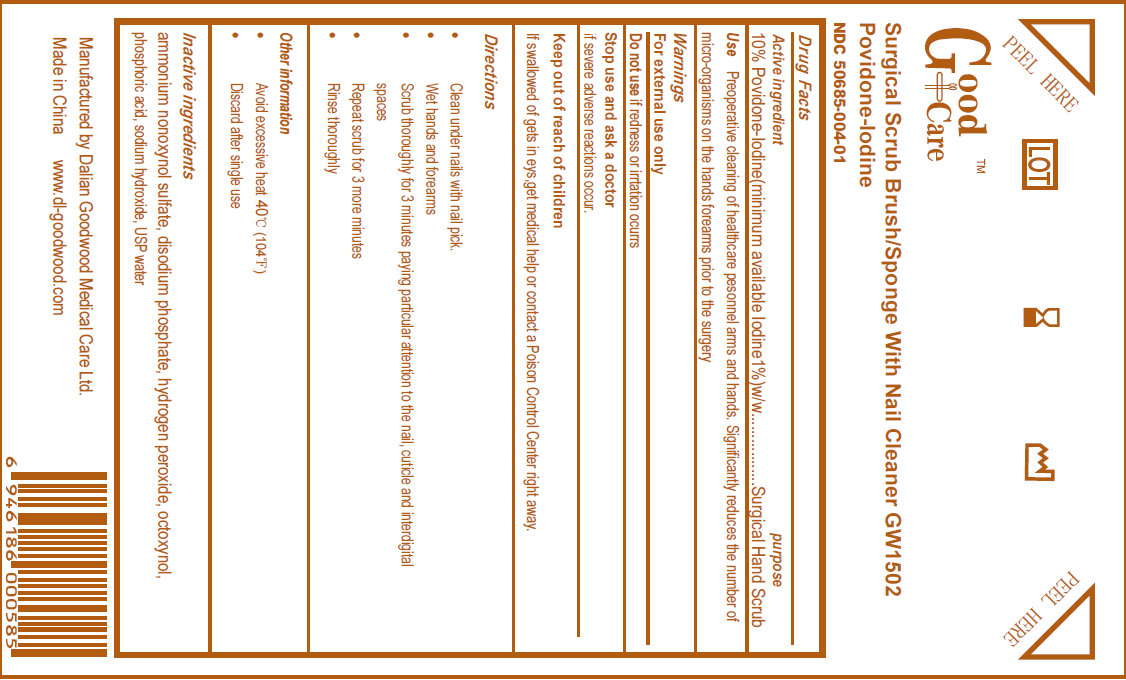
GOOD CARE SURGICAL BRUSH WITH NAIL CLEANER- povidone-iodine sponge
Dalian Goodwood Medical Care Ltd.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Surgical Scrub Brush/Sponge With Nail Cleaner Povidone Iodine
Use
Preoperative cleaning of healthcare personnel arms and hands. Significantly reduces the number of micro-organisms on the hands forearms prior to the surgery.
Keep out of reach of children. If swallowed or gets in eyes, get medical help or contact a Poison Control Center right away.
Directions
- Clean under nails with nail pick
- Wet hands and forearms
- Scrub thoroughly for 3 minutes paying particular attention to the nail, cuticle and interdigital spaces
- Repeat scrub for 3 more minutes
- Rinse thoroughly
| GOOD CARE SURGICAL BRUSH WITH NAIL CLEANER
povidone-iodine sponge |
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| Labeler - Dalian Goodwood Medical Care Ltd. (529575699) |
| Registrant - Dalian Goodwood Medical Care Ltd. (529575699) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Dalian Goodwood Medical Care Ltd. | 529575699 | manufacture(50685-004) | |
Revised: 12/2020
Document Id: b72389b0-ed37-1dce-e053-2a95a90a8193
Set id: 553f0d58-5198-49cb-beb1-cfe86d907254
Version: 4
Effective Time: 20201223
Dalian Goodwood Medical Care Ltd.