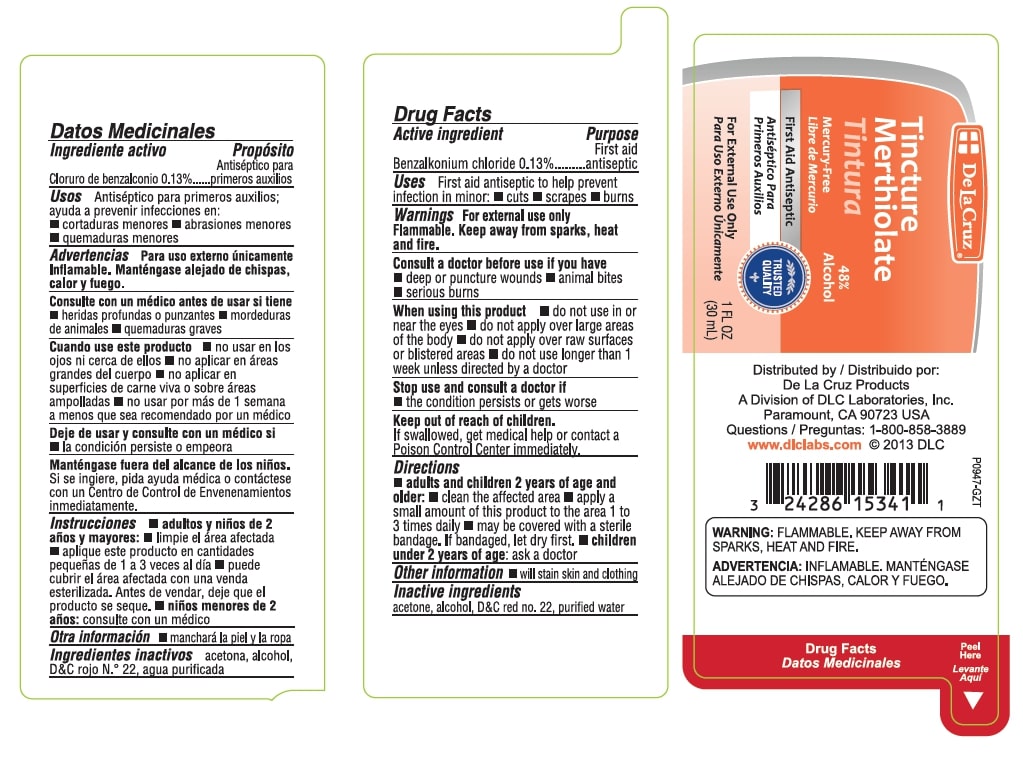
Label: TINCTURE MERTHIOLATE- benzalkonium chloride tincture
- NDC Code(s): 24286-1532-7
- Packager: DLC Laboratories, Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 18, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Uses
-
Warnings
For external use only.
Flammable. Keep away from sparks, heat and fire.
Consult a doctor before use if you have - deep or puncture wounds - animal bites - serious burns - Directions
- Other Information
- Inactive Ingredients
-
Product Labeling
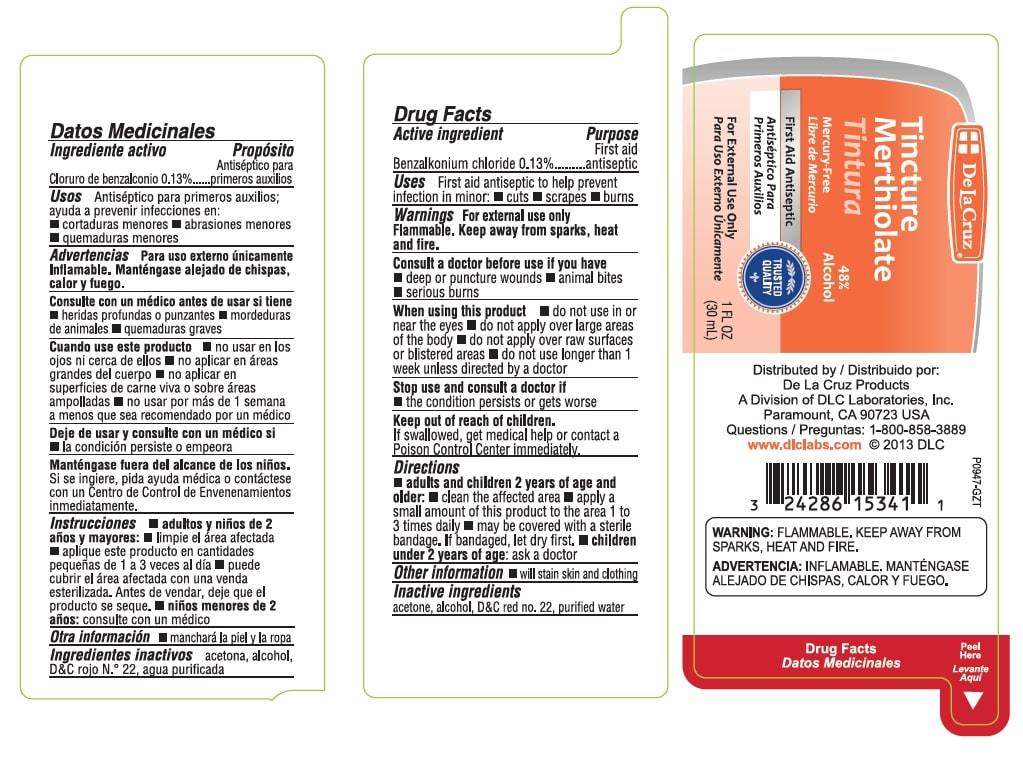
Tincture Merthiolate
48% Alcohol
Mercury Free
First Aid Antiseptic
For External Use Only
1 FL OZ (30 mL)
Distributed by
De La Cruz Products
A Division of DLC Laboratories, Inc.
Paramount, CA 90723 USA
Questions: 1-800-858-3889
www.dlclabs.comWarnings: FLAMMABLE, KEEP AWAY FROM SPARKS, HEAT AND FIRE.
-
INGREDIENTS AND APPEARANCE
TINCTURE MERTHIOLATE
benzalkonium chloride tinctureProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:24286-1532 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 1.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength ACETONE (UNII: 1364PS73AF) ALCOHOL (UNII: 3K9958V90M) D&C RED NO. 22 (UNII: 1678RKX8RT) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:24286-1532-7 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 12/13/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 03/22/2013 Labeler - DLC Laboratories, Inc (093351930) Registrant - Pharma Nobis, LLC (118564114) Establishment Name Address ID/FEI Business Operations Pharma Nobis, LLC 118564114 analysis(24286-1532) , manufacture(24286-1532) , pack(24286-1532) , label(24286-1532)