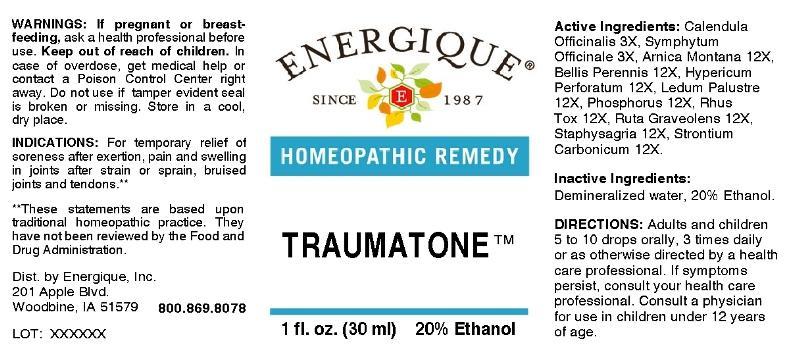
TRAUMATONE- calendula officinalis, symphytum officinale, arnica montana, bellis perennis, hypericum perforatum, ledum palustre, phosphorus, rhus tox, ruta graveolens, staphysagria, strontium carbonicum liquid
Energique, Inc.
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Drug Facts:
ACTIVE INGREDIENTS:
Calendula Officinalis 3X, Symphytum Officinale 3X, Arnica Montana 12X, Bellis Perennis 12X, Hypericum Perforatum 12X, Ledum Palustre 12X, Phosphorus 12X, Rhus Tox 12X, Ruta Graveolens 12X, Staphysagria 12X, Strontium Carbonicum 12X
INDICATIONS:
For temporary relief of soreness after exertion, pain and swelling in joints after strain or sprain, bruised joints and tendons.**
**These statements are based upon traditional homeopathic practice. They have not been reviewed by the Food and Drug Administration.
WARNINGS:
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Do not use if tamper evident seal is broken or missing. Store in a cool, dry place.
DIRECTIONS:
Adults and children 5 to 10 drops orally, 3 times daily or as otherwise directed by a health care professional. If symptoms persist, consult your health care professional. Consult a physician for use in children under 12 years of age.
KEEP OUT OF REACH OF CHILDREN:
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
| TRAUMATONE
calendula officinalis, symphytum officinale, arnica montana, bellis perennis, hypericum perforatum, ledum palustre, phosphorus, rhus tox, ruta graveolens, staphysagria, strontium carbonicum liquid |
|||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||
| Labeler - Energique, Inc. (789886132) |
| Registrant - Apotheca Company (844330915) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Apotheca Company | 844330915 | manufacture(44911-0064) , api manufacture(44911-0064) , label(44911-0064) , pack(44911-0064) | |