DEHYDRATED ALCOHOL- dehydrated alcohol injection, solution
General Injectables and Vaccines, Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Dehydrated Alcohol
DESCRIPTION
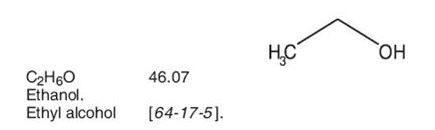
Dehydrated Alcohol Injection, USP consists of not less than 98% by volume of ethanol (ethyl alcohol). Dehydrated alcohol is hypobaric in relation to the cerebrospinal fluid. It is injected proximate to nerve tissues and into spinal subarachnoid spaces to produce degeneration of nerve function (neurolysis) for control of chronic pain. The product contains no bacteriostat or antimicrobial agent (other than ethanol) and no added buffer. Dehydrated alcohol is a clear, colorless liquid miscible with water and has the following structural formula:
CLINICAL PHARMACOLOGY
Alcohol produces injury to tissue cells by dehydration and precipitation of protoplasm. When alcohol is injected in close proximity to nerve tissues, it produces neuritis and nerve degeneration (neurolysis). Deliberate injury to selected spinal nerves, peripheral nerves, or ganglia by injection of alcohol results in more or less enduring block of sensory, motor and autonomic function.
The injection of alcohol used for therapeutic neurolysis involves amounts too small to produce significant systemic effects of ethanol.
Ninety to 98% of ethyl alcohol that enters the body is completely oxidized.
INDICATIONS AND USAGE
Dehydrated Alcohol Injection is indicated for therapeutic neurolysis of nerves or ganglia for the relief of intractable chronic pain in such conditions as inoperable cancer and trigeminal neuralgia (ticdouloureux) , in patients for whom neurosurgical procedures are contraindicated. Relief of trigerminal neuralgia usually is only temporary. Other conditions for which injection of alcohol has been reported include glossopharyngeal neuralgia, angina pectoris, and sever claudication due to peripheral vascular insufficiency.
Alcohol concentrations of 40 to 50% (prepared by appropriate dilution of dehydrated alcohol) have been used for epidural or individual motor nerve injections to control certain manifestations of cerebral palsy and spastic paraplegia. Similar concentrations also have been injected for celiac plexus block to relieve pain of inoperable upper abdominal cancer, and have been injected intra-and-subcutaneously for relief of intractable pruritus ani.
CONTRAINDICTIONS
Subarachnoid injection of dehydrated alcohol is contraindicated in patients receiving anticoagulants because of the danger of bleeding.
WARNINGS
Alcohol is a flammable liquid and should be kept cool and away from any heat source. Alcohol injections should be made with care to avoid unwanted tissue necrosis. Proper positioning of the patient is essential to control localization of injections of dehydrated alcohol (which is hypobaric) into the subarachnoid space.
PRECAUTIONS
Do not administer unless solution is clear and container is intact. Discard unused portion.
It is sometimes advisable to make a trial injection of procaine or other local anesthetic prior to alcohol injection as a means of confirming accurate placement of the needle, and to decrease pain experience during the procedure. X-ray visualization for precise placement also may be advisable.
When used for selective sensory block within the subarachnoid space, it is essential to avoid contact of the alcohol with the anterior (motor) roots of the spinal nerve to be treated if motor paralysis is not desired., when the peripheral nerves are injected, care should be taken that residual alcohol is not deposited along the needle track or in any other locations where tissue destruction is not wanted. Instances have been reported in which the pain resulting from post-injection neuritis was more sever tan that existing before the injection.
Pregnancy Category C: Animal
ADVERSE REACTIONS
The most commonly encountered side effects are post-injection neuritis with persistent pain, hyperesthesia and paresthesia. Subarachnoid neurolysis and lumbar sympathetic block may be followed by motor paralysis, bladder or rectal incontinence and impotence. Severe hypotension may follow celiac ganglion injection. Corneal anesthesia, meningitis or cranial nerve palsy may follow injection of gasserian ganglion.
OVERDOSAGE
Excessive or faulty localization of injections may result in unwanted post-injection neuritis and/or tissue necrosis. In such casees, efforts should be directed toward dilution of deposited alcohol when feasible, relief of pain with analgesics and surgical intervention if indicated.
Hypotension following celiac ganglion injection may be controlled with appropriate vasopressor agents. See PRECAUTIONS and ADVERSE REACTIONS.
DOSAGE AND ADMINISTRATION
The dosage of Dehydrated Alcohol Injection for therapeutic nerve or ganglion bllock varies from as little as 0.05 to 0.5 mL in trigeminal neuralgia to 0.5 to 1.0 mL per interspace for subarachnoid injections. Doses larger than 1.5 mL are seldom required. All injections should be made slowly and only after all steps have been taken to insure precise placement of the alcohol. A 1.0 mL tuberculin syringe is desirable to facilitate accurate measurement of the dose. Separate needles should be used for injection of successive interspaces or other sites. Since Dehydrated Alcohol Injection is hypobaric as compared to spinal fluid, proper positioning of the patient is essential to control localization of injections into the subarachnoid space.
When lesser concentratios of alcohol are used, larger volumes are usually injected. A dose of 2 mL of 45% alcohol has been used for injecting individiual motor nerves, or from 1.5 to 4.0 mL for epidural injection in children with spastic cerebral palsy; 50 mL of 50% alcohol has been used for celiac plexus blockade.
The LD50 oral dose in rats is 13.7 g/kg.
Parenteral drug products should be inspected visually for particulate matter and discorloration prior to administration, whenever solution and container permit. See PRECAUTIONS.
| DEHYDRATED ALCOHOL
dehydrated alcohol injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - General Injectables and Vaccines, Inc. (108250663) |
