CYLERT
-
pemoline tablet
CYLERT
-
pemoline tablet, chewable
AbbVie Inc.
----------
CYLERT SHOULD NOT BE USED BY PATIENTS UNTIL THERE HAS BEEN A COMPLETE DISCUSSION OF THE RISKS AND BENEFITS OF CYLERT THERAPY AND WRITTEN INFORMED CONSENT HAS BEEN OBTAINED (SEE PATIENT INFORMATION/CONSENT FORM). A SUPPLY OF PATIENT INFORMATION/ CONSENT FORMS AS PRINTED AT THE END OF THIS INSERT IS AVAILABLE, FREE OF CHARGE, BY CALLING (847) 937-7302. PERMISSION TO USE THE PATIENT INFORMATION/CONSENT FORM BY PHOTOCOPY REPRODUCTION IS HEREBY GRANTED BY ABBOTT LABORATORIES.
Because of its association with life threatening hepatic failure, CYLERT should not ordinarily be considered as first line drug therapy for ADHD (see INDICATIONS AND USAGE). Because CYLERT provides an observable symptomatic benefit, patients who fail to show substantial clinical benefit within 3 weeks of completing dose titration, should be withdrawn from CYLERT therapy.
Since CYLERT's marketing in 1975, 15 cases of acute hepatic failure have been reported to the FDA. While the absolute number of reported cases is not large, the rate of reporting ranges from 4 to 17 times the rate expected in the general population. This estimate may be conservative because of under reporting and because the long latency between initiation of CYLERT treatment and the occurrence of hepatic failure may limit recognition of the association. If only a portion of actual cases were recognized and reported, the risk could be substantially higher.
Of the 15 cases reported as of December 1998, 12 resulted in death or liver transplantation, usually within four weeks of the onset of signs and symptoms of liver failure. The earliest onset of hepatic abnormalities occurred six months after initiation of CYLERT. Although some reports described dark urine and nonspecific prodromal symptoms (e.g., anorexia, malaise, and gastrointestinal symptoms), in other reports it was not clear if any prodromal symptoms preceded the onset of jaundice.
Treatment with CYLERT should be initiated only in individuals without liver disease and with normal baseline liver function tests. It is not clear if baseline and periodic liver function testing are predictive of these instances of acute liver failure; however it is generally believed that early detection of drug-induced hepatic injury along with immediate withdrawal of the suspect drug enhances the likelihood for recovery. Accordingly, the following liver monitoring program is recommended: Serum ALT (SGPT) levels should be determined at baseline, and every two weeks thereafter. If CYLERT therapy is discontinued and then restarted, liver function test monitoring should be done at baseline and reinitiated at the frequency above.
CYLERT should be discontinued if serum ALT (SGPT) is increased to a clinically significant level, or any increase ≥ 2 times the upper limit of normal, or if clinical signs and symptoms suggest liver failure (see PRECAUTIONS).
The physician who elects to use CYLERT should obtain written informed consent from the patient prior to initiation of CYLERT therapy (see PATIENT INFORMATION/CONSENT FORM).
DESCRIPTION
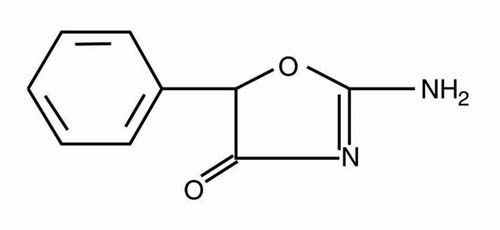
CYLERT (pemoline) is a central nervous system stimulant. Pemoline is structurally dissimilar to the amphetamines and methylphenidate.
It is an oxazolidine compound and is chemically identified as 2-amino-5-phenyl-2-oxazolin-4-one. Pemoline has the following structural formula:
Pemoline is a white, tasteless, odorless powder, relatively insoluble (less than 1 mg/mL) in water, chloroform, ether, acetone, and benzene; its solubility in 95% ethyl alcohol is 2.2 mg/mL.
CYLERT (pemoline) is supplied as tablets containing 18.75 mg, 37.5 mg or 75 mg of pemoline for oral administration. CYLERT is also available as chewable tablets containing 37.5 mg of pemoline.
Inactive Ingredients
18.75 mg tablet: corn starch, gelatin, lactose, magnesium hydroxide, polyethylene glycol and talc.
37.5 mg tablet: corn starch, FD&C Yellow No. 6, gelatin, lactose, magnesium hydroxide, polyethylene glycol and talc.
37.5 mg chewable tablet: corn starch, FD&C Yellow No. 6, magnesium hydroxide, magnesium stearate, mannitol, polyethylene glycol, povidone, talc and artificial flavor.
75 mg tablet: corn starch, gelatin, iron oxide, lactose, magnesium hydroxide, polyethylene glycol and talc.
CLINICAL PHARMACOLOGY
CYLERT (pemoline) has a pharmacological activity similar to that of other known central nervous system stimulants; however, it has minimal sympathomimetic effects. Although studies indicate that pemoline may act in animals through dopaminergic mechanisms, the exact mechanism and site of action of the drug in man is not known.
There is neither specific evidence which clearly establishes the mechanism whereby CYLERT produces its mental and behavioral effects in children, nor conclusive evidence regarding how these effects relate to the condition of the central nervous system.
Pemoline is rapidly absorbed from the gastrointestinal tract. Approximately 50% is bound to plasma proteins. The serum half-life of pemoline is approximately 12 hours. Peak serum levels of the drug occur within 2 to 4 hours after ingestion of a single dose. Multiple dose studies in adults at several dose levels indicate that steady state is reached in approximately 2 to 3 days. In animals given radiolabeled pemoline, the drug was widely and uniformly distributed throughout the tissues, including the brain.
Pemoline is metabolized by the liver. Metabolites of pemoline include pemoline conjugate, pemoline dione, mandelic acid, and unidentified polar compounds. CYLERT is excreted primarily by the kidneys with approximately 50% excreted unchanged and only minor fractions present as metabolites.
CYLERT (pemoline) has a gradual onset of action. Using the recommended schedule of dosage titration, significant clinical benefit may not be evident until the third or fourth week of drug administration.
INDICATIONS AND USAGE
CYLERT (pemoline) is indicated in Attention Deficit Hyperactivity Disorder (ADHD). Because of its association with life threatening hepatic failure, CYLERT should not ordinarily be considered as first line therapy for ADHD (see BOXED WARNING).
CYLERT (pemoline) therapy should be part of a total treatment program which typically includes other remedial measures (psychological, educational, social) for a stabilizing effect in children with a behavioral syndrome characterized by the following group of developmentally inappropriate symptoms: moderate to severe distractibility, short attention span, hyperactivity, emotional lability, and impulsivity. The diagnosis of this syndrome should not be made with finality when these symptoms are only of comparatively recent origin. Nonlocalizing (soft) neurological signs, learning disability, and abnormal EEG may or may not be present, and a diagnosis of central nervous system dysfunction may or may not be warranted.
CONTRAINDICATIONS
CYLERT (pemoline) is contraindicated in patients with known hypersensitivity or idiosyncrasy to the drug. CYLERT should not be administered to patients with impaired hepatic function (see BOXED WARNING and ADVERSE REACTIONS).
WARNINGS
Decrements in the predicted growth (i.e., weight gain and/or height) rate have been reported with the long-term use of stimulants in children. Therefore, patients requiring long-term therapy should be carefully monitored.
PRECAUTIONS
General
Clinical experience suggests that in psychotic children, administration of CYLERT may exacerbate symptoms of behavior disturbance and thought disorder.
CYLERT should be administered with caution to patients with significantly impaired renal function.
Information for Patients
Patients should be informed that CYLERT therapy has been associated with liver abnormalities ranging from reversible liver function test increases that do not cause any symptoms to liver failure, which may result in death. Patients should be informed that the risk of liver failure in the general population is relatively rare; however patients taking CYLERT are at a greater risk of developing liver failure than that expected in the general population. At present, there is no way to predict who is likely to develop liver failure; however only patients without liver disease and with normal baseline liver function tests should initiate CYLERT therapy. Patients should be advised to follow their doctors directives for liver function tests prior to and during CYLERT therapy. Patients should be advised to be alert for signs of liver dysfunction (jaundice, anorexia, gastrointestinal complaints, malaise, etc.) and to report them to their doctor immediately if they should occur.
The physician who elects to use CYLERT should obtain written informed consent from patients prior to initiation of CYLERT therapy (see PATIENT INFORMATION/CONSENT FORM.)
Laboratory Tests
Since CYLERT's market introduction, there have been reports of elevated liver enzymes associated with its use. Many of these patients had this increase detected several months after starting CYLERT. Most patients were asymptomatic, with the increase in liver enzymes returning to normal after CYLERT was discontinued.
Treatment with CYLERT should be initiated only in individuals without liver disease and with normal baseline liver function tests. It is not clear if baseline and periodic liver function testing are predictive of these instances of acute liver failure; however it is generally believed that early detection of drug-induced hepatic injury along with immediate withdrawal of the suspect drug enhances the likelihood for recovery. Accordingly, the following liver monitoring program is recommended.
Serum ALT (SGPT) levels should be determined at baseline, and every two weeks thereafter. If CYLERT therapy is discontinued and then restarted, liver function test monitoring should be done at baseline and reinitiated at the frequency above. CYLERT should be discontinued if serum ALT (SGPT) is increased to a clinically significant level, or any increase ≥ 2 times the upper limit of normal, or if clinical signs and symptoms suggest liver failure (see BOXED WARNING).
Drug Interactions
The interaction of CYLERT (pemoline) with other drugs has not been studied in humans. Patients who are receiving CYLERT concurrently with other drugs, especially drugs with CNS activity, should be monitored carefully.
Decreased seizure threshold has been reported in patients receiving CYLERT concomitantly with antiepileptic medications.
Carcinogenesis
Long-term studies have been conducted in rats with doses as high as 150 mg/kg/day for eighteen months. There was no significant difference in the incidence of any neoplasm between treated and control animals.
Mutagenesis
Data are not available concerning long-term effects on mutagenicity in animals or humans.
Impairment of Fertility
The results of studies in which rats were given 18.75 and 37.5 mg/kg/day indicated that pemoline did not affect fertility in males or females at those doses.
Pregnancy
Teratogenic Effects
Pregnancy Category B
Reproduction studies have been performed in rats and rabbits at doses of 18.75 and 37.5 mg/kg/day and have revealed no evidence of impaired fertility or harm to the fetus. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when CYLERT is administered to a nursing woman.
Pediatric Use
Safety and effectiveness in children below the age of 6 years have not been established.
Long-term effects of CYLERT in children have not been established (see WARNINGS).
CNS stimulants, including pemoline, have been reported to precipitate motor and phonic tics and Tourette's syndrome. Therefore, clinical evaluation for tics and Tourette's syndrome in children and their families should precede use of stimulant medications.
Drug treatment is not indicated in all cases of ADHD and should be considered only in light of complete history and evaluation of the child. The decision to prescribe CYLERT (pemoline) should depend on the physician's assessment of the chronicity and severity of the child's symptoms and their appropriateness for his/her age. Prescription should not depend solely on the presence of one or more of the behavioral characteristics.
Geriatric Use
Clinical studies of CYLERT did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
ADVERSE REACTIONS
The following are adverse reactions in decreasing order of severity within each category associated with CYLERT:
Hepatic
There have been reports of hepatic dysfunction, ranging from asymptomatic reversible increases in liver enzymes to hepatitis, jaundice and fatal hepatic failure, in patients taking CYLERT (see BOXED WARNING and PRECAUTIONS).
Central Nervous System
The following CNS effects have been reported with the use of CYLERT: convulsive seizures; literature reports indicate that CYLERT may precipitate attacks of Gilles de la Tourette syndrome; hallucinations; dyskinetic movements of the tongue, lips, face and extremities; abnormal oculomotor function including nystagmus and oculogyric crisis; mild depression; dizziness; increased irritability; headache; and drowsiness.
Insomnia is the most frequently reported side effect of CYLERT; it usually occurs early in therapy prior to an optimum therapeutic response. In the majority of cases it is transient in nature or responds to a reduction in dosage.
Gastrointestinal
Anorexia and weight loss may occur during the first weeks of therapy. In the majority of cases it is transient in nature; weight gain usually resumes within three to six months.
DRUG ABUSE AND DEPENDENCE
Abuse
CYLERT failed to demonstrate a potential for self-administration in primates. However, the pharmacologic similarity of pemoline to other psychostimulants with known dependence liability suggests that psychological and/or physical dependence might also occur with CYLERT. There have been isolated reports of transient psychotic symptoms occurring in adults following the long-term misuse of excessive oral doses of pemoline. CYLERT should be given with caution to emotionally unstable patients who may increase the dosage on their own initiative.
OVERDOSAGE
Signs and symptoms of acute overdosage, resulting principally from overstimulation of the central nervous system and from excessive sympathomimetic effects, may include the following: vomiting, agitation, tremors, hyperreflexia, muscle twitching, convulsions (may be followed by coma), euphoria, confusion, hallucinations, delirium, sweating, flushing, headache, hyperpyrexia, tachycardia, hypertension and mydriasis. Consult with a Certified Poison Control Center regarding treatment for up to date guidance and advice. Treatment consists of appropriate supportive measures. The patient must be protected against self-injury and against external stimuli that would aggravate overstimulation already present. Gastric contents may be evacuated by gastric lavage. Other measures to detoxify the gut include administration of activated charcoal and a cathartic. Chlorpromazine has been reported in the literature to be useful in decreasing CNS stimulation and sympathomimetic effects.
Efficacy of peritoneal dialysis or extracorporeal hemodialysis for CYLERT overdosage has not beenestablished.
DOSAGE AND ADMINISTRATION
CYLERT (pemoline) is administered as a single oral dose each morning. The recommended starting dose is 37.5 mg/day. This daily dose should be gradually increased by 18.75 mg at one week intervals until the desired clinical response is obtained. The effective daily dose for most patients will range from 56.25 to 75 mg. The maximum recommended daily dose of pemoline is 112.5 mg.
Clinical improvement with CYLERT is gradual. Using the recommended schedule of dosage titration, significant benefit may not be evident until the third or fourth week of drug administration. Because CYLERT provides an observable symptomatic benefit, patients who fail to show substantial clinical benefit within 3 weeks of completing dose titration, should be withdrawn from CYLERT therapy.
Where possible, drug administration should be interrupted occasionally to determine if there is a recurrence of behavioral symptoms sufficient to require continued therapy.
HOW SUPPLIED
CYLERT (pemoline) is supplied as monogrammed, grooved tablets in three dosage strengths:
18.75 mg white tablets (imprinted with Abbott “A” logo and the Abbo-Code TH),
Bottles of 100 (NDC 0074-6025-13).
37.5 mg orange-colored tablets (imprinted with Abbott“A” logo and the Abbo-Code TI),
Bottles of 100 (NDC 0074-6057-13).
75 mg tan-colored tablets (imprinted with Abbott“A” logo and the Abbo-Code TJ),
Bottles of 100 (NDC 0074-6073-13).
CYLERT (pemoline) Chewable is supplied as 37.5 mg monogrammed, grooved orange-colored tablets (imprinted with Abbott“A” logo and the Abbo-Code TK),
Bottles of 100 (NDC 0074-6088-13).
PATIENT INFORMATION/CONSENT FORM
Cylert® (pemoline) should not be used by patients until there has been a complete discussion of the risks and benefits of Cylert therapy and written informed consent has been obtained.
IMPORTANT INFORMATION
Cylert therapy has been associated with liver abnormalities ranging from reversible liver function test increases that do not cause any symptoms to liver failure, which may result in death. Therefore, you should have a full discussion of the risks and benefits of Cylert before beginning therapy.
PATIENT CONSENT
My (son, daughter, ward)___________________________'s treatment with Cylert has been explained to me by Dr.______________.
The following points of information, among others, have been specifically discussed and explained and I have had the opportunity to ask any questions concerning this information.
- I, ________(Patient/Parent/Guardian's name), understand that Cylert is used to treat certain types of patients with the behavioral syndrome called attention deficit hyperactivity disorder (ADHD) and that I (my son/daughter/ward) am that type of patient.
Initials:_________ - I understand that there is a risk that I (my son/daughter/ward) might develop liver failure, which may result in death, while taking Cylert. I understand that this could occur even after long-term therapy.
Initials:_________ - I understand that I (my son/daughter/ward) should have blood taken to test liver function before Cylert is begun, and every two weeks from then on while taking Cylert. I understand that although the liver function tests may help detect if I (my son/daughter/ward) develop liver damage, it may do so only after significant, irreversible and potentially fatal damage has already occurred.
Initials:_________ - I understand that if I (my son/daughter/ward) stop taking Cylert and then restart it at a later time (e.g., after summer vacation), I (my son/daughter/ward) should again have blood taken to test liver function before Cylert is restarted, and every two weeks from then on while taking Cylert.
Initials:_________ - I understand that I should immediately report any unusual symptoms to the doctor and should be especially aware of persistent nausea, vomiting, fatigue, lethargy, loss of appetite, abdominal pain, dark urine, or yellowing of the skin or eyes.
Initials:_________
I now authorize Dr._____________________ to begin my (son/daughter/ward's) treatment with Cylert, or if treatment with Cylert has already begun, to continue this treatment.
_______________________________________
_______________________________________
PHYSICIAN STATEMENT
I have fully explained to the patient (parent/guardian), _______________________ the nature and purpose of treatment with Cylert and the potential risks associated with that treatment. I have asked if he/she has any questions regarding this treatment or the associated risks and have answered these questions to the best of my ability.
_______________________________________
NOTE TO PHYSICIAN: It is strongly recommended that you retain a completed copy of this informed consent form in your patient's records.
SUPPLY OF PATIENT INFORMATION/CONSENT FORMS: A supply of Patient Information/Consent Forms as printed above is available, free of charge, by calling (847) 937-7302. Permission to use the above Patient Information/Consent Form by photocopy reproduction is hereby granted by Abbott Laboratories.
| CYLERT
pemoline tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA016832 | 01/27/1975 | 04/30/2008 |
| CYLERT
pemoline tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA016832 | 01/27/1975 | 04/30/2008 |
| CYLERT
pemoline tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA016832 | 01/27/1975 | 04/30/2008 |
| CYLERT
pemoline tablet, chewable |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA017703 | 01/30/1976 | 04/30/2008 |
| Labeler - AbbVie Inc. (078458370) |
Revised: 01/2013 AbbVie Inc.