ALPRAZOLAM
- alprazolam tablet
Lake Erie Medical DBA Quality Care Products LLC
----------
Description Section
DESCRIPTION
Alprazolam is a triazolo analog of the 1,4 benzodiazepine class of central nervous system-active compounds. The chemical name of alprazolam is 8-Chloro-1-methyl-6-phenyl-4H-s-triazolo [4,3-α] [1,4] benzodiazepine.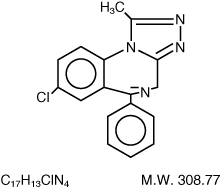
Alprazolam is a white to off-white crystalline powder, which is soluble in alcohol but which has no appreciable solubility in water at physiological pH.
Each alprazolam tablet, for oral administration, contains 0.25, 0.5, or 1 mg of alprazolam. Inactive ingredients: docusate sodium, lactose monohydrate, magnesium stearate, microcrystalline cellulose, pregelatinized starch, and sodium benzoate. Additionally, the 0.5 mg also contains FD and C Yellow #6 Aluminum Lake, and the 1 mg also contains FD and C Blue #2 Aluminum Lake.
Alprazolam tablets are contraindicated in patients with known sensitivity to this drug or other benzodiazepines. Alprazolam may be used in patients with open angle glaucoma who are receiving appropriate therapy, but is contraindicated in patients with acute narrow angle glaucoma.
Alprazolam is contraindicated with ketoconazole and itraconazole, since these medications significantly impair the oxidative metabolism mediated by cytochrome P450 3A (CYP3A) (see WARNINGS and PRECAUTIONS: Drug Interactions).
Side effects to alprazolam tablets, if they occur, are generally observed at the beginning of therapy and usually disappear upon continued medication. In the usual patient, the most frequent side effects are likely to be an extension of the pharmacological activity of alprazolam, eg, drowsiness or light-headedness.
The data cited in the two tables below are estimates of untoward clinical event incidence among patients who participated under the following clinical conditions: relatively short duration (ie, four weeks) placebo-controlled clinical studies with dosages up to 4 mg/day of alprazolam (for the management of anxiety disorders or for the short-term relief of the symptoms of anxiety) and short-term (up to ten weeks) placebo-controlled clinical studies with dosages up to 10 mg/day of alprazolam in patients with panic disorder, with or without agoraphobia.
These data cannot be used to predict precisely the incidence of untoward events in the course of usual medical practice where patient characteristics, and other factors often differ from those in clinical trials. These figures cannot be compared with those obtained from other clinical studies involving related drug products and placebo as each group of drug trials are conducted under a different set of conditions.
Comparison of the cited figures, however, can provide the prescriber with some basis for estimating the relative contributions of drug and non-drug factors to the untoward event incidence in the population studied. Even this use must be approached cautiously, as a drug may relieve a symptom in one patient but induce it in others. (For example, an anxiolytic drug may relieve dry mouth [a symptom of anxiety] in some subjects but induce it [an untoward event] in others.)
Additionally, for anxiety disorders the cited figures can provide the prescriber with an indication as to the frequency with which physician intervention (eg, increased surveillance, decreased dosage or discontinuation of drug therapy) may be necessary because of the untoward clinical event.
ANXIETY DISORDERS
*Events reported by 1% or more of alprazolam patients are included.
†None reported
Treatment-Emergent
Symptom Incidence*
Incidence of
Intervention
Because of Symptom
Alprazolam Placebo Alprazolam
Number of Patients 565 505 565
% of Patients Reporting:
Central Nervous System
Drowsiness 41.0 21.6 15.1
Light-headedness 20.8 19.3 1.2
Depression 13.9 18.1 2.4
Headache 12.9 19.6 1.1
Confusion 9.9 10.0 0.9
Insomnia 8.9 18.4 1.3
Nervousness 4.1 10.3 1.1
Syncope 3.1 4.0 †
Dizziness 1.8 0.8 2.5
Akathisia 1.6 1.2 †
Tiredness/Sleepiness † † 1.8
Gastrointestinal
Dry Mouth 14.7 13.3 0.7
Constipation 10.4 11.4 0.9
Diarrhea 10.1 10.3 1.2
Nausea/Vomiting 9.6 12.8 1.7
Increased Salivation 4.2 2.4 †
Cardiovascular
Tachycardia/Palpitations 7.7 15.6 0.4
Hypotension 4.7 2.2 †
Sensory
Blurred Vision 6.2 6.2 0.4
Musculoskeletal
Rigidity 4.2 5.3 †
Tremor 4.0 8.8 0.4
Cutaneous
Dermatitis/Allergy 3.8 3.1 0.6
Other
Nasal Congestion 7.3 9.3 †
Weight Gain 2.7 2.7 †
Weight Loss 2.3 3.0 †
In addition to the relatively common (i.e., greater than 1%) untoward events enumerated in the table above, the following adverse events have been reported in association with the use of benzodiazepines: dystonia, irritability, concentration difficulties, anorexia, transient amnesia or memory impairment, loss of coordination, fatigue, seizures, sedation, slurred speech, jaundice, musculoskeletal weakness, pruritus, diplopia, dysarthria, changes in libido, menstrual irregularities, incontinence and urinary retention.
Treatment-Emergent Adverse Events Reported in Placebo-Controlled Trials of Panic Disorder
*Events reported by 1% or more of alprazolam patients are included.
PANIC DISORDER
Treatment-Emergent
Symptom Incidence*
Alprazolam Placebo
Number of Patients 1388 1231
% of Patients Reporting:
Central Nervous System
Drowsiness 76.8 42.7
Fatigue and Tiredness 48.6 42.3
Impaired Coordination 40.1 17.9
Irritability 33.1 30.1
Memory Impairment 33.1 22.1
Light-headedness/Dizziness 29.8 36.9
Insomnia 29.4 41.8
Headache 29.2 35.6
Cognitive Disorder 28.8 20.5
Dysarthria 23.3 6.3
Anxiety 16.6 24.9
Abnormal Involuntary Movement 14.8 21.0
Decreased Libido 14.4 8.0
Depression 13.8 14.0
Confusional State 10.4 8.2
Muscular Twitching 7.9 11.8
Increased Libido 7.7 4.1
Change in Libido (Not Specified) 7.1 5.6
Weakness 7.1 8.4
Muscle Tone Disorders 6.3 7.5
Syncope 3.8 4.8
Akathisia 3.0 4.3
Agitation 2.9 2.6
Disinhibition 2.7 1.5
Paresthesia 2.4 3.2
Talkativeness 2.2 1.0
Vasomotor Disturbances 2.0 2.6
Derealization 1.9 1.2
Dream Abnormalities 1.8 1.5
Fear 1.4 1.0
Feeling Warm 1.3 0.5
Gastrointestinal
Decreased Salivation 32.8 34.2
Constipation 26.2 15.4
Nausea/Vomiting 22.0 31.8
Diarrhea 20.6 22.8
Abdominal Distress 18.3 21.5
Increased Salivation 5.6 4.4
Cardio-Respiratory
Nasal Congestion 17.4 16.5
Tachycardia 15.4 26.8
Chest Pain 10.6 18.1
Hyperventilation 9.7 14.5
Upper Respiratory Infection 4.3 3.7
Sensory
Blurred Vision 21.0 21.4
Tinnitus 6.6 10.4
Musculoskeletal
Muscular Cramps 2.4 2.4
Muscle Stiffness 2.2 3.3
Cutaneous
Sweating 15.1 23.5
Rash 10.8 8.1
Other
Increased Appetite 32.7 22.8
Decreased Appetite 27.8 24.1
Weight Gain 27.2 17.9
Weight Loss 22.6 16.5
Micturition Difficulties 12.2 8.6
Menstrual Disorders 10.4 8.7
Sexual Dysfunction 7.4 3.7
Edema 4.9 5.6
Incontinence 1.5 0.6
Infection 1.3 1.7
In addition to the relatively common (ie, greater than 1%) untoward events enumerated in the table above, the following adverse events have been reported in association with the use of alprazolam: seizures, hallucinations, depersonalization, taste alterations, diplopia, elevated bilirubin, elevated hepatic enzymes, and jaundice.
Panic disorder has been associated with primary and secondary major depressive disorders and increased reports of suicide among untreated patients (see PRECAUTIONS: General).
n a larger database comprised of both controlled and uncontrolled studies in which 641 patients received alprazolam, discontinuation-emergent symptoms which occurred at a rate of over 5% in patients treated with alprazolam and at a greater rate than the placebo treated group were as follows:
DISCONTINUATION-EMERGENT SYMPTOM INCIDENCE
Percentage of 641 Alprazolam-Treated
Panic Disorder Patients Reporting Events
Body System/Event
Neurologic Gastrointestinal
Insomnia 29.5 Nausea/Vomiting 16.5
Light-headedness 19.3 Diarrhea 13.6
Abnormal involuntary movement 17.3 Decreased salivation 10.6
Headache 17.0 Metabolic-Nutritional
Muscular twitching 6.9 Weight loss 13.3
Impaired coordination 6.6 Decreased appetite 12.8
Muscle tone disorders 5.9
Weakness 5.8 Dermatological
Psychiatric Sweating 14.4
Anxiety 19.2
Fatigue and Tiredness 18.4 Cardiovascular
Irritability 10.5 Tachycardia 12.2
Cognitive disorder 10.3
Memory impairment 5.5 Special Senses
Depression 5.1 Blurred vision 10.0
Confusional state 5.0
From the studies cited, it has not been determined whether these symptoms are clearly related to the dose and duration of therapy with alprazolam in patients with panic disorder. There have also been reports of withdrawal seizures upon rapid decrease or abrupt discontinuation of alprazolam tablets (see WARNINGS).
To discontinue treatment in patients taking alprazolam, the dosage should be reduced slowly in keeping with good medical practice. It is suggested that the daily dosage of alprazolam be decreased by no more than 0.5 mg every three days (see DOSAGE AND ADMINISTRATION). Some patients may benefit from an even slower dosage reduction. In a controlled postmarketing discontinuation study of panic disorder patients which compared this recommended taper schedule with a slower taper schedule, no difference was observed between the groups in the proportion of patients who tapered to zero dose; however, the slower schedule was associated with a reduction in symptoms associated with a withdrawal syndrome.
As with all benzodiazepines, paradoxical reactions such as stimulation, increased muscle spasticity, sleep disturbances, hallucinations and other adverse behavioral effects such as agitation, rage, irritability, and aggressive or hostile behavior have been reported rarely. In many of the spontaneous case reports of adverse behavioral effects, patients were receiving other CNS drugs concomitantly and/or were described as having underlying psychiatric conditions. Should any of the above events occur, alprazolam should be discontinued. Isolated published reports involving small numbers of patients have suggested that patients who have borderline personality disorder, a prior history of violent or aggressive behavior, or alcohol or substance abuse may be at risk for such events. Instances of irritability, hostility, and intrusive thoughts have been reported during discontinuation of alprazolam in patients with posttraumatic stress disorder.
Various adverse drug reactions have been reported in association with the use of alprazolam since market introduction. The majority of these reactions were reported through the medical event voluntary reporting system. Because of the spontaneous nature of the reporting of medical events and the lack of controls, a causal relationship to the use of alprazolam cannot be readily determined. Reported events include: liver enzyme elevations, hepatitis, hepatic failure, Stevens-Johnson syndrome, hyperprolactinemia, gynecomastia, and galactorrhea.
Dosage should be individualized for maximum beneficial effect. While the usual daily dosages given below will meet the needs of most patients, there will be some who require doses greater than 4 mg/day. In such cases, dosage should be increased cautiously to avoid adverse effects.
Treatment for patients with anxiety should be initiated with a dose of 0.25 to 0.5 mg given three times daily. The dose may be increased to achieve a maximum therapeutic effect, at intervals of 3 to 4 days, to a maximum daily dose of 4 mg, given in divided doses. The lowest possible effective dose should be employed and the need for continued treatment reassessed frequently. The risk of dependence may increase with dose and duration of treatment.
In all patients, dosage should be reduced gradually when discontinuing therapy or when decreasing the daily dosage. Although there are no systematically collected data to support a specific discontinuation schedule, it is suggested that the daily dosage be decreased by no more than 0.5 mg every three days. Some patients may require an even slower dosage reduction.
The successful treatment of many panic disorder patients has required the use of alprazolam at doses greater than 4 mg daily. In controlled trials conducted to establish the efficacy of alprazolam in panic disorder, doses in the range of 1 to 10 mg daily were used. The mean dosage employed was approximately 5 to 6 mg daily. Among the approximately 1700 patients participating in the panic disorder development program, about 300 received alprazolam in dosages of greater than 7 mg/day, including approximately 100 patients who received maximum dosages of greater than 9 mg/day. Occasional patients required as much as 10 mg a day to achieve a successful response.
In elderly patients, in patients with advanced liver disease or in patients with debilitating disease, the usual starting dose is 0.25 mg, given two or three times daily. This may be gradually increased if needed and tolerated. The elderly may be especially sensitive to the effects of benzodiazepines. If side effects occur at the recommended starting dose, the dose may be lowered.
Alprazolam tablets, USP for oral administration are available as:
0.25 mg: Oval, white tablets debossed GG 256 on one side and scored on the reverse side and supplied as:
0.5 mg: Oval, peach tablets debossed GG 257 on one side and scored on the reverse side and supplied as:
49999-039-30
49999-039-60
49999-039-90
1 mg: Oval, blue tablets debossed GG 258 on one side and scored on the reverse side and supplied as:
Store at 20°-25°C (68°-77°F) (see USP Controlled Room Temperature).
Dispense in a tight, light-resistant container.
ANIMAL STUDIES
When rats were treated with alprazolam at 3, 10, and 30 mg/kg/day (15 to 150 times the maximum recommended human dose) orally for 2 years, a tendency for a dose related increase in the number of cataracts was observed in females and a tendency for a dose related increase in corneal vascularization was observed in males. These lesions did not appear until after 11 months of treatmen
| ALPRAZOLAM
alprazolam tablet |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Lake Erie Medical DBA Quality Care Products LLC (831276758) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Lake Erie Medical DBA Quality Care Products LLC | 831276758 | repack(49999-032) | |
