ORAVIG- miconazole tablet
Par Pharmaceuticals Companies, Inc.
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ORAVIG safely and effectively. See full prescribing information for ORAVIG.
ORAVIG (miconazole) buccal tablets Initial U.S. Approval: 1974 INDICATIONS AND USAGEORAVIG is an azole antifungal indicated for the local treatment of oropharyngeal candidiasis in adults ( 1). DOSAGE AND ADMINISTRATIONDOSAGE FORMS AND STRENGTHS50 mg buccal tablets ( 3). CONTRAINDICATIONSKnown hypersensitivity to miconazole, milk protein concentrate, or any other component of the product ( 4). WARNINGS AND PRECAUTIONSHypersensitivity reactions: Anaphylactic reactions have been reported in patients receiving miconazole. Discontinue ORAVIG immediately at the first sign of hypersensitivity ( 5.1). ADVERSE REACTIONSMost common adverse reactions (≥2%) are: diarrhea, headache, nausea, dysgeusia, upper abdominal pain, and vomiting ( 6.1). To report SUSPECTED ADVERSE REACTIONS, contact Par Pharmaceutical, Inc. at 1-800-828-9393 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONSWarfarin: Miconazole may enhance anticoagulant effect. Monitor prothrombin time, INR, and watch for bleeding ( 7.1). USE IN SPECIFIC POPULATIONSSee 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling. Revised: 06/2013 |
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
ORAVIG is indicated for the local treatment of oropharyngeal candidiasis (OPC) in adults.
2 DOSAGE AND ADMINISTRATION
2.1 Basic Dosing Information
The recommended dosing schedule for ORAVIG is the application of one 50 mg buccal tablet to the upper gum region (canine fossa) once daily for 14 consecutive days.
2.2 Administration Instructions
ORAVIG should be applied in the morning, after brushing the teeth. The tablet should be applied with dry hands. The rounded side surface of the tablet should be placed against the upper gum just above the incisor tooth (canine fossa) and held in place with slight pressure over the upper lip for 30 seconds to ensure adhesion. The tablet is round on one side for comfort, but either side of the tablet can be applied to the gum.
Once applied, ORAVIG stays in position and gradually dissolves. [ See Clinical Pharmacology (12.3)] Subsequent applications of ORAVIG should be made to alternate sides of the mouth. Before applying the next tablet, the patient should clear away any remaining tablet material. In addition,
- •
- ORAVIG should not be crushed, chewed or swallowed.
- •
- Food and drink can be taken normally when ORAVIG is in place but chewing gum should be avoided.
- •
- If ORAVIG does not adhere or falls off within the first 6 hours, the same tablet should be repositioned immediately. If the tablet still does not adhere, a new tablet should be placed.
- •
- If ORAVIG is swallowed within the first 6 hours, the patient should drink a glass of water and a new tablet should be applied only once.
- •
- If ORAVIG falls off or is swallowed after it was in place for 6 hours or more, a new tablet should not be applied until the next regularly scheduled dose. [See Patient Counseling Information (17)].
3 DOSAGE FORMS AND STRENGTHS
ORAVIG is a buccal tablet containing 50 mg of miconazole. ORAVIG tablets are round, off-white tablets, with a rounded side and a flat side. The tablets are marked with an “L” on the flat side.
4 CONTRAINDICATIONS
ORAVIG is contraindicated in patients with known hypersensitivity (e.g., anaphylaxis) to miconazole, milk protein concentrate, or any other component of the product.
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity
Allergic reactions, including anaphylactic reactions and hypersensitivity, have been reported with the administration of miconazole products, including ORAVIG. Discontinue ORAVIG immediately at the first sign of hypersensitivity.
There is no information regarding cross-hypersensitivity between miconazole and other azole antifungal agents. Monitor patients with a history of hypersensitivity to azoles.
6 ADVERSE REACTIONS
The following serious adverse drug reactions are discussed in detail in other sections of labeling:
- •
- Hypersensitivity reactions [see Warnings and Precautions (5.1)]
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The overall safety of ORAVIG was assessed in 480 adult subjects: 315 HIV-infected subjects, 147 subjects with head and neck cancer, and 18 healthy subjects.
HIV Infected Patients
Two trials were conducted in immunocompromised HIV infected patients: one randomized, double-blind, double-dummy, active-controlled design (N = 290 ORAVIG, 287 control) and one non-comparative trial (N = 25).
In the randomized, double blind trial (Study 1), 290 HIV infected subjects used ORAVIG once daily for 14 days, and 287 subjects used 10 mg clotrimazole troches five times daily for 14 days. Adverse reactions occurring in ≥ 2% of patients in either treatment are presented in Table 1.
|
(MedDRA v 9.1 System Organ Class and Preferred Term)Adverse Reaction |
N = 290 (%)ORAVIG |
N = 287 (%)Clotrimazole troches |
|
Patients with any adverse reaction during the study |
158 (54.5) |
146 (50.9) |
|
Abdominal pain upperGastrointestinal disorders Diarrhea Nausea Vomiting Dry mouth |
1.725.9 9.06.63.82.8 |
2.823.7 8.07.73.11.7 |
|
GastroenteritisInfections and infestations Upper respiratory infection |
1.415.9 2.1 |
2.817.1 2.4 |
|
Ageusia Nervous system disorders Headache |
2.413.1 7.6 |
0.38.4 6.6 |
|
NeutropeniaBlood and lymphatic disorders Anemia Lymphopenia |
0.76.9 2.81.7 |
2.18.4 1.72.1 |
|
PainGeneral disorders and administration site conditions Fatigue |
1.06.6 2.8 |
2.88.0 2.1 |
|
Pharyngeal painRespiratory/thoracic Cough |
0.75.2 2.8 |
2.47.7 1.7 |
|
Increased GGTInvestigations |
1.05.5 |
2.86.3 |
Overall local adverse reactions, including oral discomfort, oral burning, oral pain, gingival pain, gingival swelling, gingival pruritis, tongue ulceration, mouth ulceration, glossodynia, dry mouth, application site pain or discomfort, toothache, loss of taste, and altered taste, were reported by 35 (12.1%) patients who received miconazole buccal tablet compared to 27 (9.4%) patients who received clotrimazole troches.
Head and Neck Cancer Patients
In the randomized, open-label comparative trial of oropharyngeal candidiasis in patients with head and neck cancer who had received radiation therapy (Study 2), 147 patients used ORAVIG once daily for 14 days and 147 patients used 125 mg of miconazole oral gel four times daily for 14 days. Adverse reactions occurring in ≥2% of patients in either arm are listed in Table 2.
|
(MedDRA v 9.1 System Organ Class and Preferred Term) Adverse Reaction |
N = 147 (%)ORAVIG |
N = 147 (%)Miconazole gel |
|
Patients with at least one adverse reaction |
30 (20.4) |
32 (21.8) |
|
GlossodyniaGastrointestinal disorders Abdominal pain, upper Oral discomfort Nausea Vomiting |
08.8 1.42.70.70.7 |
2.013.6 2.02.72.72.0 |
|
DysgeusiaNervous system disorders |
4.15.4 |
01.4 |
|
PruritusSkin and subcutaneous |
2.03.4 |
0.70.7 |
Overall local adverse reactions, including oral discomfort, oral pain, dry mouth, glossodynia, loss of taste, altered taste, tongue ulceration, mouth ulceration, tooth disorder, and application site discomfort or pain, were experienced by 14 (9.5%) patients who used ORAVIG compared to 16 (10.9%) patients who used miconazole gel.
Overall ORAVIG Safety Experience In Patients and Healthy Subjects
Adverse reactions reported in the overall safety database of 480 subjects who received miconazole buccal tablet is listed in Table 3.
|
(MedDRA v 9.1 System Organ Class and Preferred Term) Adverse reaction |
N = 480 (%)ORAVIG |
|
Patients with at least one AE |
209 (43.5) |
|
VomitingGastrointestinal disorders Diarrhea Nausea Abdominal pain upper |
2.520.6 6.04.62.5 |
|
Infections and infestations |
11.9 |
|
DysgeusiaNervous system disorders Headache |
2.910.6 5.0 |
Discontinuation of ORAVIG due to adverse drug reactions occurred in 0.6% overall.
7 DRUG INTERACTIONS
7.1 Warfarin
Concomitant administration of miconazole and warfarin has resulted in enhancement of anticoagulant effect. Cases of bleeding and bruising following the concomitant use of warfarin and topical, intravaginal, or oral miconazole were reported. Closely monitor prothrombin time, International Normalized Ratio (INR), or other suitable anticoagulation tests if ORAVIG is administered concomitantly with warfarin. Also monitor for evidence of bleeding.
7.2 Drugs Metabolized Through CYP2C9 and 3A4
No formal drug interaction studies have been performed with ORAVIG. Miconazole is a known inhibitor of CYP2C9 and CYP3A4. Although the systemic absorption of miconazole following ORAVIG administration is minimal and plasma concentrations of miconazole are substantially lower than when given intravenously, the potential for interaction with drugs metabolized through CYP2C9 and CYP3A4 such as oral hypoglycemics, phenytoin, or ergot alkaloids cannot be ruled out.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C
There are no adequate and well-controlled clinical trials of ORAVIG in pregnant women. ORAVIG should not be used during pregnancy unless the potential benefit to the mother outweighs the potential risk to the fetus.
Miconazole nitrate administered orally at doses of 80 mg/kg/day or higher to pregnant rats or rabbits crossed the placenta and resulted in embryo- and fetotoxicity, including increased fetal resorptions. These doses also resulted in prolonged gestation and dystocia in rats, but not in rabbits. Embryofetotoxicity was not observed in intravenous studies with miconazole at lower doses of 40 mg/kg/day in rats and 20 mg/kg/day in rabbits, which are approximately 8 times higher than the dose a patient would receive if she swallowed an ORAVIG buccal tablet, based on body surface area comparisons. Teratogenicity was not reported in any animal study with miconazole.
8.3 Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when ORAVIG is administered to a nursing woman.
8.4 Pediatric Use
Safety and effectiveness of ORAVIG in pediatric patients below the age of 16 years have not been established. The ability of pediatric patients to comply with the application instructions has not been evaluated. Use in younger children is not recommended due to potential risk of choking.
8.5 Geriatric Use
Clinical studies of ORAVIG did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects.
10 OVERDOSAGE
Overdose with miconazole in humans has not been reported in the literature.
Miconazole absorption and systemic exposure following application of ORAVIG are minimal [ see Clinical Pharmacology (12.3)].
Symptomatic and supportive care is the basis for management.
11 DESCRIPTION
ORAVIG (miconazole) buccal tablets are applied topically to the gum once daily and release miconazole as the buccal tablet gradually dissolves [ see Clinical Pharmacology (12.3)].
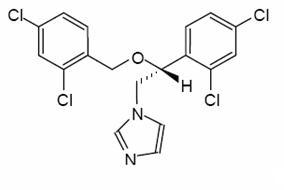
Miconazole is an imidazole antifungal agent and is described chemically as 1-[(2RS)-2-[(2,4-dichlorobenzyl)oxy]-2-(2,4-dichlorophenyl)ethyl]-1H-imidazole with an empirical formula of C 18H14Cl4N2O and a molecular weight of 416.13. The structural formula is shown in Figure 1.
Miconazole drug substance is a white to almost white powder.
ORAVIG contains 50 mg of miconazole base, USP and the following inactive ingredients: hypromellose, USP; milk protein concentrate; corn starch, NF; lactose monohydrate, NF; sodium lauryl sulfate, NF; magnesium stearate, NF; and talc, USP.
12 CLINICAL PHARMACOLOGY
12.3 Pharmacokinetics
Absorption and Distribution
Salivary
Single dose application of ORAVIG containing 50 mg of miconazole to the buccal mucosa of 18 healthy volunteers provided mean maximum salivary concentrations of 15 mcg/mL at 7 hours after application of the tablet. This provided an average saliva exposure to miconazole estimated from the AUC (0-24h) of 55.23 mcg⋅h/mL. The pharmacokinetic parameters of miconazole in the saliva of healthy volunteers are provided in Table 4.
| * Median | |
|
Salivary PK Parameters (N = 18) |
Mean ± SD
|
|
AUC0-24h (mcg⋅h/mL) |
55.2 ± 35.1 |
|
Cmax (mcg/mL) |
15.1 ± 16.2 |
|
Tmax (hour) |
7* |
In healthy volunteers, the duration of buccal adhesion was on average 15 hours following a single dose application of ORAVIG 50 mg.
Plasma
Plasma concentrations of miconazole were below the lower limit of quantification (0.4 mcg/mL) in 157/162 (97%) samples from healthy volunteers following single-dose application of ORAVIG 50 mg. Measurable plasma concentrations ranged from 0.5 to 0.83 mcg/mL.
Plasma concentrations of miconazole evaluated after 7 days of treatment in 40 HIV-positive patients were all below the limit of quantification (0.1 mcg/mL).
Metabolism and Excretion
Most of the absorbed miconazole is metabolized by the liver with less than 1% of the administered dose found unchanged in urine. In healthy volunteers, the terminal half-life is 24 hours following systemic administration. There are no active metabolites of miconazole.
Food Effect
There was no formal food effect study conducted with ORAVIG; however, in clinical studies patients were allowed to eat and drink while taking ORAVIG.
12.4 Microbiology
Mechanism of Action
Miconazole inhibits the enzyme cytochrome P450 14α-demethylase which leads to inhibition of ergosterol synthesis, an essential component of the fungal cell membrane. Miconazole also affects the synthesis of triglycerides and fatty acids and inhibits oxidative and peroxidative enzymes, increasing the amount of reactive oxygen species within the cell.
Activity in vitro and in vivo
Miconazole is active against Candida albicans, C. parapsilosis, and C. tropicalis. Correlation between minimum inhibitory concentration (MIC) results in vitro and clinical outcome has yet to be established.
Drug Resistance
In vitro studies have shown that some Candida strains that demonstrate reduced susceptibility to one antifungal azole may also exhibit reduced susceptibility to other azoles suggesting cross-resistance.
Clinically relevant resistance to systemically utilized triazoles may occur in Candida species. Resistance may occur by multiple mechanisms such as changes in amino acids and/or in the regulation of the target enzyme and of a variety of efflux pump proteins. Multiple mechanisms may co-exist in the same isolate. Resistance breakpoints, correlating in vitro activity with clinical efficacy, have not been established for miconazole.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies with miconazole have not been conducted.
Miconazole nitrate was not genotoxic when tested in vitro in a bacterial reverse mutation (Ames) assay or in an in vivo mouse bone marrow micronucleus test. Intraperitoneal injections of miconazole to mice induced chromosomal aberrations in spermatocytes and bone marrow cells, and morphologic abnormalities in sperm at doses similar to or below clinical doses. However, no impairment of fertility was observed in intravenous studies with miconazole at 40 mg/kg/day in rats or 20 mg/kg/day in rabbits, which are approximately 8 times higher than the dose a patient would receive if she swallowed an ORAVIG buccal tablet, based on body surface area comparisons.
14 CLINICAL STUDIES
Study in HIV Infected Patients
The efficacy and safety of ORAVIG in the treatment of OPC was evaluated in a randomized, double-blind, double-dummy, multicenter trial comparing ORAVIG 50 mg once daily for 14 consecutive days (n = 290) with clotrimazole troches 10 mg 5 times per day for 14 days (n = 287) in HIV-positive patients with OPC. Seventy-five percent of patients were not receiving highly active antiretroviral treatment, 5% had CD4+ cell count < 50 cells/mm 3, and 17% had a history of previous OPC. The mean viral load was 117,000 copies/mL. Patients were required to have symptoms and microbiological documentation of OPC for study entry. Most of the infections were caused by C. albicans (85%), followed by C. tropicalis (9%), and C. parapsilosis (3%). About 2% of the subjects were infected with more than one Candida species.
Clinical cure [defined as a complete resolution of both signs and symptoms of OPC at the test of cure (TOC) visit (days 17-22)], and clinical relapse by days 35-38 (21-24 days after end of therapy) are presented in Table 5. Mycological cure [defined as eradication (i.e., no yeast isolates) of Candida species] at the TOC visit (days 17-22) is also reported in the table.
| Analysis population includes all randomized patients who took at least 1 dose of study medication. One randomized subject excluded from the ORAVIG arm. In those subjects who relapsed, the mean time to relapse was 15.3 days (SD 4.6) and 15.7 days (SD 6.6), in the ORAVIG and Clotrimazole treatment arms, respectively.† Difference in clinical cure rates (ORAVIG-clotrimazole troche) was -4.5%, with a 95% CI: (-12.4%, 3.4%).‡ Percentage based on those who had clinical cure. | ||
|
ORAVIG |
Clotrimazole troches |
|
|
Clinical cure† |
176 (60.7%) |
187 (65.2%) |
|
Clinical relapse‡ | ||
|
Yesb |
48 (27.3%) |
52 (27.8%) |
|
No |
124 (70.5%) |
133 (71.1%) |
|
Missing |
4 (2.3%) |
2 (1.1%) |
|
Mycological cure |
79 (27.2%) |
71 (24.7%) |
Study in Head and Neck Cancer Patients
The efficacy and safety of ORAVIG 50 mg was evaluated in an open-label, randomized, multicenter trial comparing ORAVIG 50 mg once daily for 14 days to miconazole oral gel 125 mg four times daily for 14 days in head and neck cancer patients who had received radiation therapy. Most of the infections were caused by C. albicans (71%), and C. tropicalis (8%). About 7% of the subjects were infected with more than one Candida species. Success rates of treatment at day 14 [defined as a complete (complete disappearance of candidiasis lesions) or partial response (improvement by at least 2 points of the score for extent of oral lesion compared with the score at day 1) based on a blind assessment] are shown in Table 6. Also reported in Table 6 are relapse rate at day 30, and mycologic cure assessed at day 14.
| Analysis population includes all subjects who received at least one dose of study medication. Reasons for not receiving treatment included negative mycological culture, informed consent withdrawn, or lost during screening. Six patients excluded per arm. CR: complete response; PR: partial response In those subjects who relapsed, the mean time to relapse was 18.8 days (SD 16.3) and 20.6 days (SD 13.5), in the ORAVIG and Miconazole oral gel group, respectively.† Difference in clinical complete response rates (ORAVIG-Miconazole oral gel) was 6.2%, with a 95% CI: (-5.2%, 17.6%).‡ Percentage based on those who had complete response. | |||||
|
ORAVIG |
Miconazole oral gel |
||||
|
Success rate (CR+PR)b |
79 (53.4%) |
69 (46.6%) |
|||
|
CR† |
74 (50.0%) |
64 (43.8%) |
|||
|
Clinical relapse‡ | |||||
|
Yesc |
14 (18.9%) |
8 (12.5%) |
|||
|
No |
59 (79.7%) |
56 (87.5%) |
|||
|
Missing |
1 (1.4%) |
0 |
|||
|
Mycological cure |
66 (44.6%) |
78 (53.4%) |
|||
16 HOW SUPPLIED/STORAGE AND HANDLING
ORAVIG 50 mg buccal tablets are supplied as off-white tablets containing 50 mg of miconazole. ORAVIG tablets have a rounded side and a flat side. ORAVIG tablets are packaged in bottles of 14 tablets (NDC 49884-082-26).
ORAVIG should be stored at 20 to 25°C (68 to 77 °F) [see USP controlled room temperature]; excursions between 15 and 30°C permitted at room temperature. Protect from moisture, and keep out of reach of children.
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling.
17.1 Instructions for Use
The tablet should be used immediately after removal from the bottle.
- •
- Instruct patients not to crush, chew, or swallow the tablet.
- •
- The rounded side of the tablet should be applied to the upper gum above the incisor tooth in the morning, after brushing the teeth.
- •
- The tablet should be held in place for 30 seconds with a slight pressure of the finger over the upper lip to make the tablet stick to the gum.
- •
- The tablet may be used if it sticks to the cheek, inside of the lip or the gum.
- •
- If the tablet does not adhere, it should be repositioned.
- •
- As the ORAVIG tablet absorbs moisture from the mouth, it will slowly dissolve over time and should be left in place—there is no need to remove the tablet.
- •
- Subsequent applications of ORAVIG should be made to alternate sides of the gum.
- •
- If ORAVIG does not stick or falls off within the first 6 hours, the same tablet should be repositioned immediately. If the tablet does not adhere, a new tablet should be placed.
- •
- If ORAVIG is swallowed within the first 6 hours, the patient should drink a glass of water and a new tablet should be applied only once.
- •
- If ORAVIG falls off or is swallowed after it was in place for 6 hours or more, a new tablet should not be applied until the next, regularly scheduled dose.
Patients should avoid situations that could interfere with the sticking of the tablet including:
- •
- touching or pressing the tablet after placement
- •
- wearing upper denture
- •
- chewing gum
- •
- hitting tablet when brushing teeth
- •
- rinsing mouth too vigorously
17.2 Hypersensitivity and Other Adverse Reactions
Patients who develop hives, skin rash, or other symptoms of an allergic reaction, and patients who develop swelling or pain, at the tablet application site should stop ORAVIG and contact a healthcare provider. Patients may experience other adverse reactions including diarrhea, headache, nausea, and change in taste.
FDA-Approved Patient Labeling
Oravig (OR-a-vig)
(miconazole)
Buccal Tablets
Read the Patient Information that comes with ORAVIG before you start taking it and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your doctor or about your medical condition or your treatment.
What is ORAVIG?
ORAVIG is a prescription antifungal medicine used in adults to treat fungal (yeast) infections of the mouth and the throat.
It is not known if ORAVIG is safe and effective in children under the age of 16 years. It is not known if children can follow the instructions what to do with the buccal tablet. In younger children, there is a possible risk of choking.
Who should not use ORAVIG?
Do not use ORAVIG if you:
- •
- are allergic to miconazole (M-Zole, Monistat, Vusion)
- •
- are allergic to milk protein concentrate
- •
- are allergic to any of the ingredients in ORAVIG. See the end of this Patient Information leaflet for a complete list of ingredients in ORAVIG
What should I tell my doctor before using ORAVIG?
Before taking ORAVIG, tell your doctor if you:
- •
- have liver problems
- •
- have any other medical conditions
- •
- are pregnant or plan to become pregnant. It is not known if ORAVIG will harm your unborn baby. Talk to your doctor if you are pregnant or plan to become pregnant.
- •
- are breast-feeding or plan to breast-feed. It is not known if ORAVIG passes into your breast milk. Talk to your doctor about the best way to feed your baby if you use ORAVIG.
Tell your doctor about all the medicines you take, including prescription and nonprescription medicines, vitamins, and herbal supplements.
ORAVIG may affect the way other medicines work, and other medicines may affect how ORAVIG works.
Especially tell your doctor if you take:
- •
- a diabetes medicine
- •
- phenytoin (Dilantin, Phenytek)
- •
- an ergot medicine. Ask your doctor or pharmacist if you are not sure if your medicine is an ergot medicine.
- •
- the blood thinner medicine warfarin sodium (Coumadin, Jantoven)
Know the medicines you take. Keep a list of them to show your doctor and pharmacist when you get a new medicine.
How should I use ORAVIG?
- •
- Always use ORAVIG exactly as your doctor tells you. ORAVIG is usually applied in the morning after you brush your teeth.
- •
- ORAVIG is placed 1 time each day to your upper gum for 14 days.
- •
- You may eat and drink while using ORAVIG.
- •
- Do not crush, chew or swallow ORAVIG.
- •
- You should change where you place ORAVIG, between the left and right side of your upper gum with each use.
- •
- It is okay if ORAVIG sticks to your cheek, the inside of your lip or your gum. If ORAVIG does not stick or falls off of your gum within the first 6 hours, re-apply it. If it still does not stick, replace it with a new tablet.
- •
- If you swallow ORAVIG within the first 6 hours of placing, drink a glass of water and place a new ORAVIG to your gum.
- •
- If ORAVIG falls off or is swallowed after it was in place for 6 hours or more, do not apply a new ORAVIG. Just place your next dose at your regular time.
- •
- Check to see if ORAVIG is still in place after you brush your teeth, rinse your mouth, eat, or drink.
How to use ORAVIG?
- •
- Before applying the tablet,
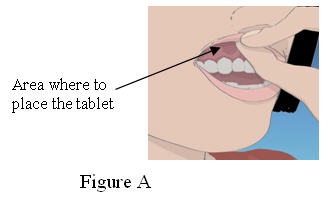
1. Locate the area on the upper gum, just above either the left or the right incisor. The incisor tooth is the tooth just to the right or left of your two front teeth (See Figure A)
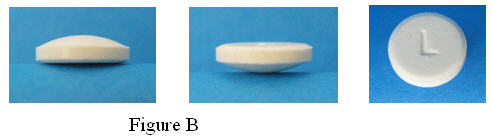
2. Take one ORAVIG tablet out of the bottle. ORAVIG is round on one side and flat on the other side (Figure B). The tablet is marked with an “L” on the flat side.
Applying the ORAVIG tablet,
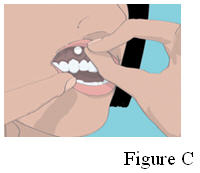
3. Place the flat side of the ORAVIG tablet on your dry fingertip. Gently push the rounded side of the tablet against your upper gum in the area shown in Figure C. Push the ORAVIG tablet up as high as it will go on your gum. The flat side will be facing the inside of your lip.
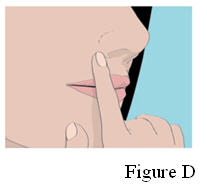
4. Hold the ORAVIG tablet in place by applying a slight pressure with your finger on the outside of your upper lip for 30 seconds. This will make the tablet stick to your gum (See Figure D).
5. Leave the tablet in place until it dissolves.
6. Before applying your next dose, be sure to clear away any remaining ORAVIG tablet material.
What should I avoid while using ORAVIG?
You should avoid activities that may prevent ORAVIG from sticking to your gum, including:
- •
- touching or pressing ORAVIG after placement
- •
- wearing upper denture that interfere with placement of the tablet
- •
- chewing-gum.
- •
- hitting tablet when brushing your teeth
- •
- rinsing your mouth too vigorously
What are the possible side effects of ORAVIG?
ORAVIG may cause serious side effects including:
Allergic reactions.Tell your doctor or get emergency medical help right away if you have any of the symptoms below:
- •
- skin rash or hives
- •
- swelling of your face, eyes, lips, tongue or throat
- •
- trouble swallowing or breathing
The most common side effects of ORAVIG include:
- •
- diarrhea
- •
- change in taste
- •
- headache
- •
- upper stomach (abdominal) pain
- •
- nausea
- •
- vomiting
Tell your doctor if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of ORAVIG. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store ORAVIG?
- •
- Store ORAVIG between 68 to 77 °F (20 to 25°C)
- •
- Keep ORAVIG dry
Keep ORAVIG and all medicine out of the reach of children.
General information about the safe and effective use of ORAVIG
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information Leaflet. Do not use ORAVIG for a condition for which it was not prescribed. Do not give ORAVIG to other people, even if they have the same symptoms that you have. It may harm them. This Patient Information Leaflet summarizes the most important information about ORAVIG. If you would like more information, talk with your doctor. You can ask your pharmacist or doctor for information about ORAVIG that is written for health professionals. You can also visit www.oravig.com for more information.
What are the ingredients in ORAVIG?
Active ingredient: miconazole
Inactive ingredients: hypromellose, milk protein concentrate, corn starch, lactose monohydrate, sodium lauryl sulfate, magnesium stearate, and talc.
Manufactured By:
Catalent Germany Schorndorf GmbH
Steinbeisstraße 2
73614 Schorndorf
Germany
Distributed By:
Strativa Pharmaceuticals, a Division of Par Pharmaceutical, Inc.
Spring Valley, NJ 10977
Revised 2/2011
US patent numbers: 6,916,485
©2011 Par Pharmaceutical, Inc.
| ORAVIG
miconazole tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Par Pharmaceuticals Companies, Inc. (092733690) |
| Registrant - Catalent Germany Schorndorf GMBH (315732628) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Catalent Germany Schorndorf GMBH | 315732628 | MANUFACTURE(49884-082) | |