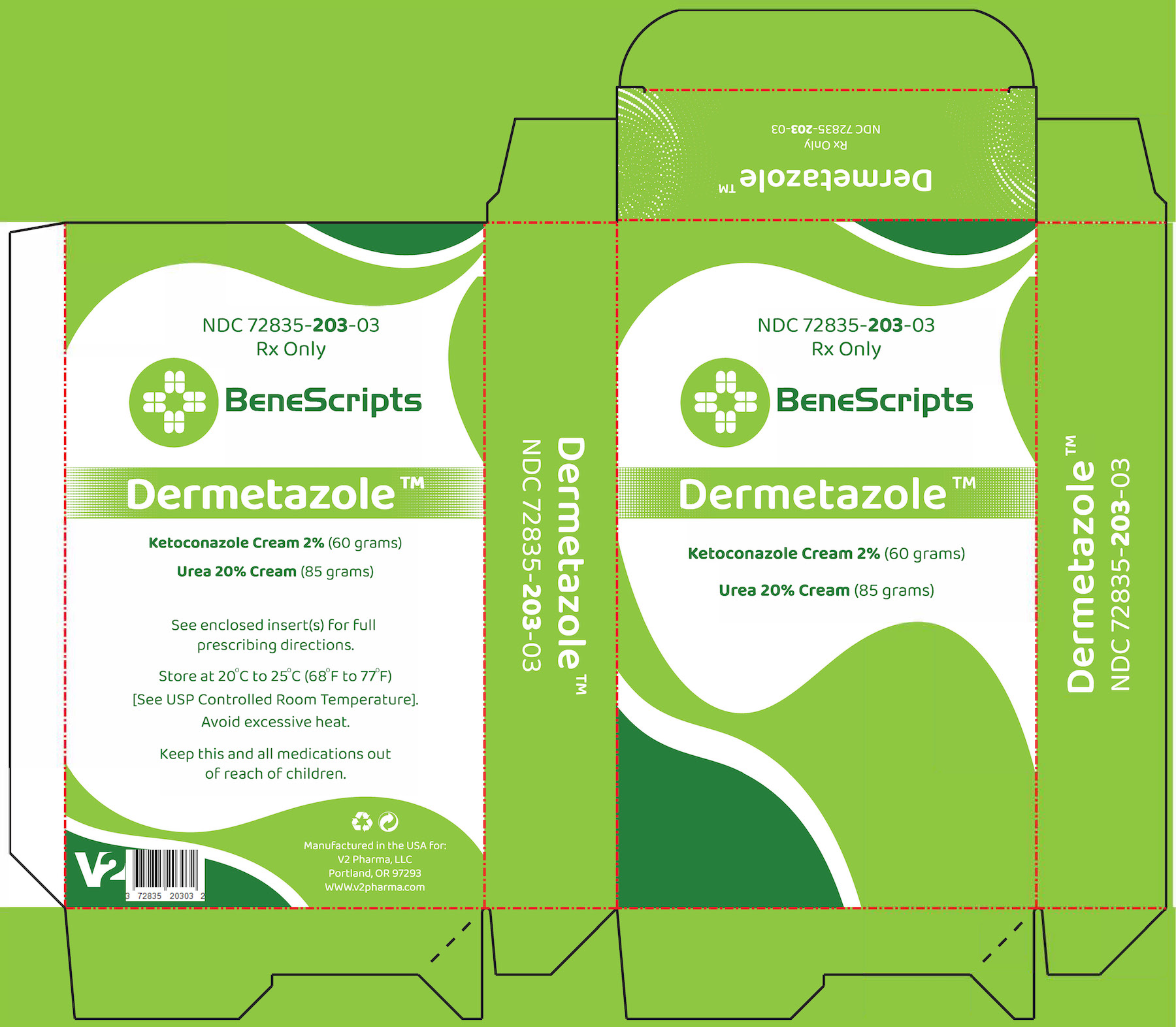
DERMETAZOLE- ketoconazole 2% and urea 20%
V2 Pharma, LLC
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Dermetazole
DERMETAZOLE DESCRIPTION
DERMETAZOLE is supplied as 3 components in a kit:
-2 TUBES OF KETOCONAZOLE CREAM 2%, 30g (60g TOTAL IN KIT) (NDC 51672-1298-2), UREA 20% CREAM, 85g (NDC 58980-610-30)
INDICATION AND USAGE
For the topical treatment of tinea corporis, tinea cruris and tinea pedis caused by Trichophyton rubrum, T. mentagrophytes and Epidermophyton floccosum; in the treatment of tinea (pityriasis) versicolor caused by Malassezia furfur (Pityrosporum orbiculare); in the treatment of cutaneous candidiasis caused by Candida spp. and in the treatment of seborrheic dermatitis. Keratolytic1
- 1
- This statement has not been evaluated by the FDA. This product is not intended to diagnose, treat, cure, or prevent disease.
DOSAGE AND ADMINISTRATION
First apply ketoconazole cream, 2% to cover the affected and immediate surrounding area. Then apply Urea 20% cream and rub into skin until completely absorbed. Apply twice a day or as directed by your physician.
WARNINGS
FOR EXTERNAL USE ONLY. Avoid contact with eyes, lips or mucous membranes. Do not use on areas of broken skin.
CONTRAINDICATIONS
Do not use if known hypersensitivity to any of the listed ingredients of any of the components included on the kit.
PRECAUTIONS
Stop use and ask a doctor if redness or irritation develops. Keep this and all other medications out of the reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
DESCRIPTION
Ketoconazole cream, 2% contains the broad-spectrum synthetic antifungal agent, ketoconazole 2%, formulated in an aqueous cream vehicle consisting of butylated hydroxyanisole (BHA), cetyl alcohol, isopropyl myristate, polysorbate 60, polysorbate 80, propylene glycol, purified water, sorbitan monostearate and stearyl alcohol.
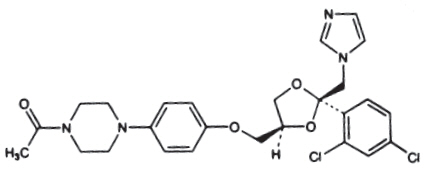
Ketoconazole is cis-1-acetyl-4-[4-[[2-(2,4-dichlorophenyl)-2-(1H-imidazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl] piperazine and has the following structural formula:
Molecular Formula: C26H28Cl2N4O4
Molecular Weight: 531.43
CLINICAL PHARMACOLOGY
When ketoconazole cream, 2% was applied dermally to intact or abraded skin of beagle dogs for 28 consecutive days at a dose of 80 mg, there were no detectable plasma levels using an assay method having a lower detection limit of 2 ng/mL.
After a single topical application to the chest, back and arms of normal volunteers, systemic absorption of ketoconazole was not detected at the 5 ng/mL level in blood over a 72-hour period.
Two dermal irritancy studies, a human sensitization test, a phototoxicity study and a photoallergy study conducted in 38 male and 62 female volunteers showed no contact sensitization of the delayed hypersensitivity type, no irritation, no phototoxicity and no photoallergenic potential due to ketoconazole cream, 2%.
Microbiology
Ketoconazole is a broad spectrum synthetic antifungal agent which inhibits the in vitro growth of the following common dermatophytes and yeasts by altering the permeability of the cell membrane: dermatophytes: Trichophyton rubrum, T. mentagrophytes, T. tonsurans, Microsporum canis, M. audouini, M. gypseum and Epidermophyton floccosum; yeasts: Candida albicans, Malassezia ovale (Pityrosporum ovale) and C. tropicalis; and the organism responsible for tinea versicolor, Malassezia furfur (Pityrosporum orbiculare). Only those organisms listed in the INDICATIONS AND USAGE section have been proven to be clinically affected. Development of resistance to ketoconazole has not been reported.
Mode of Action
In vitro studies suggest that ketoconazole impairs the synthesis of ergosterol, which is a vital component of fungal cell membranes. It is postulated that the therapeutic effect of ketoconazole in seborrheic dermatitis is due to the reduction of M. ovale, but this has not been proven.
INDICATIONS AND USAGE
Ketoconazole cream, 2% is indicated for the topical treatment of tinea corporis, tinea cruris and tinea pedis caused by Trichophyton rubrum, T. mentagrophytes and Epidermophyton floccosum; in the treatment of tinea (pityriasis) versicolor caused by Malassezia furfur (Pityrosporum orbiculare); in the treatment of cutaneous candidiasis caused by Candida spp. and in the treatment of seborrheic dermatitis.
CONTRAINDICATIONS
Ketoconazole cream, 2% is contraindicated in persons who have shown hypersensitivity to the active or excipient ingredients of this formulation.
PRECAUTIONS
General
If a reaction suggesting sensitivity or chemical irritation should occur, use of the medication should be discontinued. Hepatitis (1:10,000 reported incidence) and, at high doses, lowered testosterone and ACTH induced corticosteroid serum levels have been seen with orally administered ketoconazole; these effects have not been seen with topical ketoconazole.
Carcinogenesis, Mutagenesis, Impairment of Fertility
A long-term feeding study in Swiss Albino mice and in Wistar rats showed no evidence of oncogenic activity. The dominant lethal mutation test in male and female mice revealed that single oral doses of ketoconazole as high as 80 mg/kg produced no mutation in any stage of germ cell development. The Ames' salmonella microsomal activator assay was also negative.
Pregnancy
Teratogenic effects
Pregnancy Category C
Ketoconazole has been shown to be teratogenic (syndactylia and oligodactylia) in the rat when given orally in the diet at 80 mg/kg/day, (10 times the maximum recommended human oral dose). However, these effects may be related to maternal toxicity, which was seen at this and higher dose levels.
There are no adequate and well-controlled studies in pregnant women. Ketoconazole should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
It is not known whether Ketoconazole cream, 2% administered topically could result in sufficient systemic absorption to produce detectable quantities in breast milk. Nevertheless, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
ADVERSE REACTIONS
During clinical trials 45 (5.0%) of 905 patients treated with ketoconazole cream, 2% and 5 (2.4%) of 208 patients treated with placebo reported side effects consisting mainly of severe irritation, pruritus and stinging. One of the patients treated with ketoconazole cream developed a painful allergic reaction.
In worldwide postmarketing experience, rare reports of contact dermatitis have been associated with ketoconazole cream or one of its excipients, namely propylene glycol.
DOSAGE AND ADMINISTRATION
Cutaneous candidiasis, tinea corporis, tinea cruris, tinea pedis, and tinea (pityriasis) versicolor
It is recommended that ketoconazole cream, 2% be applied once daily to cover the affected and immediate surrounding area. Clinical improvement may be seen fairly soon after treatment is begun; however, candidal infections and tinea cruris and corporis should be treated for two weeks in order to reduce the possibility of recurrence.
Patients with tinea versicolor usually require two weeks of treatment. Patients with tinea pedis require six weeks of treatment.
Mfd. by: Taro Pharmaceuticals Inc., Brampton, Ontario, Canada L6T 1C1
Dist. by: Taro Pharmaceuticals U.S.A., Inc., Hawthorne, NY 10532
Revised: March, 2014
PK-2925-4
354
PRINCIPAL DISPLAY PANEL - 30 g Tube Carton
NDC 51672-1298-2
30 g
Ketoconazole
Cream 2%
FOR DERMATOLOGIC USE ONLY.
NOT FOR OPHTHALMIC USE.
Rx only
Keep this and all medications out of the reach of children.
TARO

Inactive Ingredients
Carbomer, Fragrance, Isopropyl Myristate, Isopropyl Palmitate, Propylene Glycol, Purified Water, Sodium Laureth Sulfate, Stearic Acid, Trolamine and Xanthan Gum.
Warnings
FOR EXTERNAL USE ONLY. Avoid contact with eyes, lips or mucous membranes. Do not use on areas of broken skin. Do not use if known hypersensitivity to any of the listed ingredients.
Directions
Apply to the affected areas twice a day or as directed by a physician. Rub into the skin until completely absorbed.
| DERMETAZOLE
ketoconazole 2% and urea 20% kit |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - V2 Pharma, LLC (102457346) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| V2 Pharma, LLC | 102457346 | label(72835-203) | |