CAREONE- psyllium husk capsule
FOODHOLD USA
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
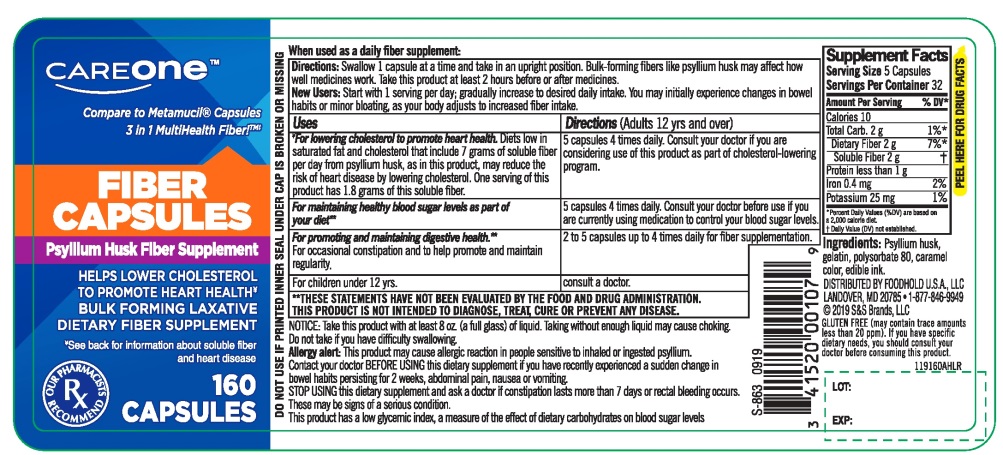
CAREONE Fiber Capsules Psyllium Husk 160 Capsules
Warnings
Choking
Taking this product without adequate fluid may cause it to swell and block your throat or esophagus and may cause choking. Do not take this product if you have difficulty in swallowing. If you experience chest pain, vomiting, or difficulty in swallowing or breathing after taking this product, seek immediate medical attention.
Allergy alert
This product may cause allergic reaction in people sensitive to inhaled or ingested psyllium.
Ask a doctor before use if you have
- ▪
- a sudden change in bowel habits persisting for 2 weeks
- ▪
- abdominal pain, nausea or vomiting
Stop use and ask a doctor if
- ▪
- constipation lasts more than 7 days
- ▪
- rectal bleeding occurs
These may be signs of a serious condition.
Directions
- ▪
- Take this product with at least 8 ounces (a full glass) of water or other fluid. Taking this product without enough liquid may cause choking. See choking warning.
- ▪
- Adults 12 years and older: 5 capsules with at least 8 oz. of liquid (swallow 1 capsule at a time) at the first sign of irregularity. Can be taken up to 3 times daily. Generally produces effect in 12-72 hours.
- ▪
- children 12 years and under: Ask a doctor
- ▪
- Laxatives, including bulk fibers, may affect how well other medicines work. If you are taking a prescription medication by mouth, take this product at least 2 hours before or 2 hours after the prescribed medicine.
- ▪
- As your body adjusts to increased fiber intake, you may experience changes in bowel habits or minor bloating.
Other information
- ▪
- each capsule contains: potassium 5 mg
- ▪
- capsules are sealed using a band around the middle
- ▪
- store at room temperature. Keep the container tightly closed to protect from humidity
- ▪
- contains a 100% natural, therapeutic fiber
- ▪
- tamper evident: do not use if printed inner seal under cap is broken or missing
PRINCIPAL DISPLAY PANEL - 160 Capsules Label
NDC 41520-907-60
Compare to Metamucil® Capsules‡
FIBER CAPSULES
Psyllium Husk Fiber Supplement
100% natural Psyllium Husk
BULK FORMING LAXATIVE
DIETARY FIBER SUPPLEMENT
160 CAPSULES
OUR PHARMACISTS RECOMMEND
|
**THESE STAEMENTS HAVE NOT BEEN EVALUATED BY FOOD AND DRUG ADMINSTRATION, THIS PRODUCT IS NOT INTEDED TO DIAGNOSE, TREAT, CURE OR PREVENT ANY DISEASE |
GLUTEN FREE (may contain trace amount less than 20 ppm). If you have specific dietary needs, you should dietary needs; you should consult your doctor before consuming this product)
DISTRIBUTED BY FOODHOLD U.S.A. LLC
LANDOVER, MD 20785
1-877-846-9949
2018 S&S Brands, LLC
Quality guaranteed or your money back.
*This product is not manufactured or distributed by Procter & Gamble, the distributor of Metamucil® 3 in1 MultiHealth Fiber!™.
| CAREONE
psyllium husk capsule |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - FOODHOLD USA (809183973) |