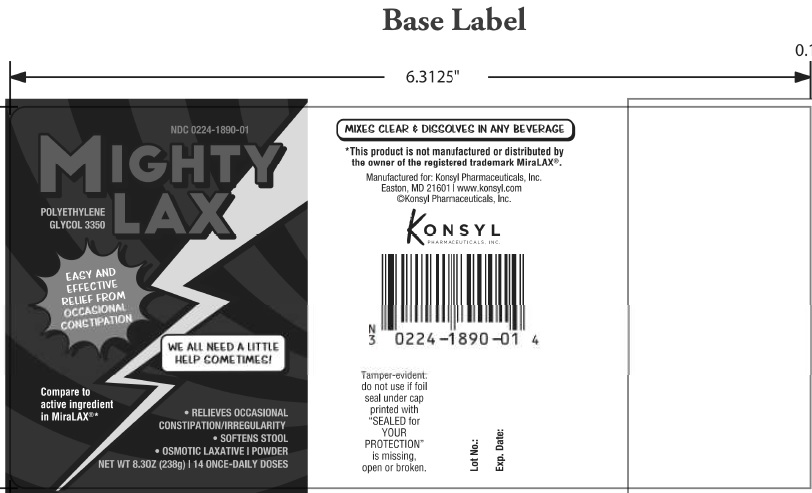
MIGHTY LAX- polyethylene glycol 3350 powder
Konsyl Pharmaceuticals, Inc.
----------
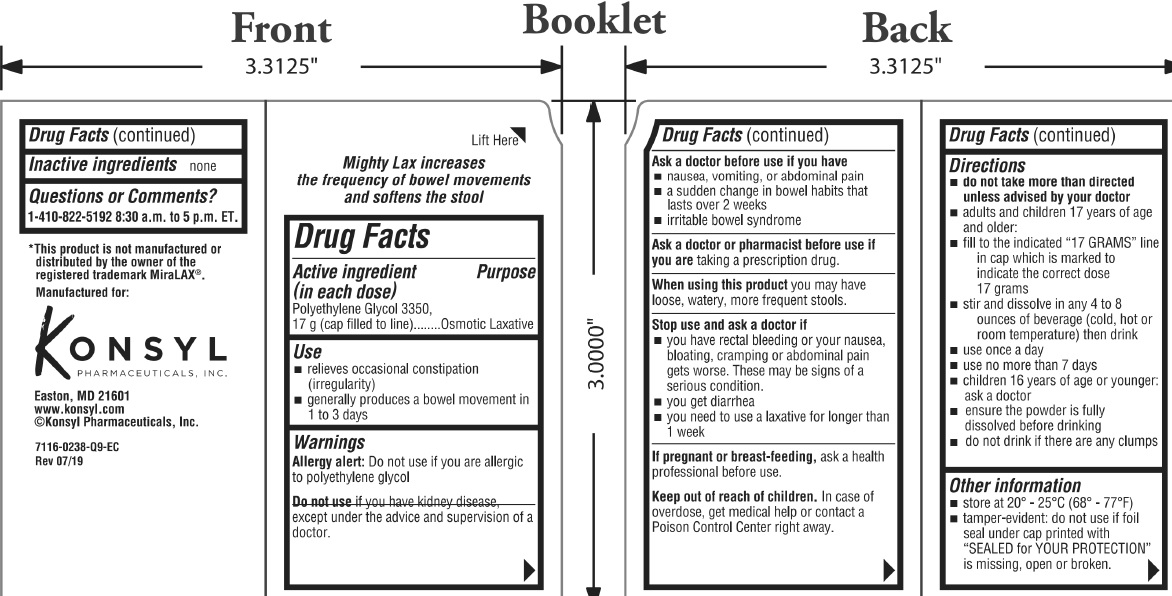
Mighty Lax
Use
- relieves occasional constipation (irregularity)
- generally produces a bowel movement in 1 to 3 days
Warnings
Allergy alert: Do not use if you are allergic to polyethylene glycol
Directions
- do not take more than directed unless advised by your doctor
- adults and children 17 years of age and older:
- fill to the indicated "17 GRAMS" line in cap which is marked to indicate the correct dose 17 grams
- stir and dissolve in any 4 to 8 ounces of beverage (cold, hot or room temperature) then drink
- use once a day
- use on more than 7 days
- children 16 years or age or younger: ask a doctor
- ensure the powder is fully dissolved before drinking
- do not drink if there are any clumps
| MIGHTY LAX
polyethylene glycol 3350 powder |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Konsyl Pharmaceuticals, Inc. (102463866) |
Revised: 12/2020
Document Id: b78c7b17-c001-41b4-e053-2995a90a04cb
Set id: 3e82de78-2f6f-47a6-b390-2be50b7a7a99
Version: 2
Effective Time: 20201228
Konsyl Pharmaceuticals, Inc.