Label: sufenta- sufentanil citrate solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 11098-050-01, 11098-050-02, 11098-050-05 - Packager: Taylor Pharmaceuticals
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CII
Drug Label Information
Updated July 2, 2008
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- N/A - Section Title Not Found In Database
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
SUFENTA® (sufentanil citrate) is a potent opioid analgesic chemically designated as N-[4-(methyoxymethyl)-1-[2-(2-thienyl)ethyl]-4-piperidinyl]-N-phenylpropanamide:2-hydroxy-1,2,3-propanetricarboxylate (1:1) with a molecular weight of 578.68. The structural formula of SUFENTA is:
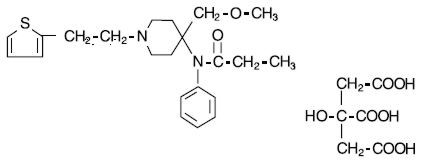
SUFENTA is a sterile, preservative free, aqueous solution containing sufentanil citrate equivalent to 50 mcg per mL of sufentanil base for intravenous and epidural injection. The solution has a pH range of 3.5 to 6.0.
-
CLINICAL PHARMACOLOGY
Pharmacology
SUFENTA is an opioid analgesic. When used in balanced general anesthesia, SUFENTA has been reported to be as much as 10 times as potent as fentanyl. When administered intravenously as a primary anesthetic agent with 100% oxygen, SUFENTA is approximately 5 to 7 times as potent as fentanyl.
Assays of histamine in patients administered SUFENTA have shown no elevation in plasma histamine levels and no indication of histamine release.
(See dosage chart for more complete information on the intravenous use of SUFENTA.)
Pharmacodynamics
Intravenous use
At intravenous doses of up to 8 mcg/kg, SUFENTA is an analgesic component of general anesthesia; at intravenous doses ≥8 mcg/kg, SUFENTA produces a deep level of anesthesia. SUFENTA produces a dose related attenuation of catecholamine release, particularly norepinephrine.
At intravenous dosages of ≥8 mcg/kg, SUFENTA produces hypnosis and anesthesia without the use of additional anesthetic agents. A deep level of anesthesia is maintained at these dosages, as demonstrated by EEG patterns. Dosages of up to 25 mcg/kg attenuate the sympathetic response to surgical stress. The catecholamine response, particularly norepinephrine, is further attenuated at doses of SUFENTA of 25 to 30 mcg/kg, with hemodynamic stability and preservation of favorable myocardial oxygen balance.
SUFENTA has an immediate onset of action, with relatively limited accumulation. Rapid elimination from tissue storage sites allows for relatively more rapid recovery as compared with equipotent dosages of fentanyl. At dosages of 1 to 2 mcg/kg, recovery times are comparable to those observed with fentanyl; at dosages of >2 to 6 mcg/kg, recovery times are comparable to enflurane, isoflurane and fentanyl. Within the anesthetic dosage range of 8 to 30 mcg/kg of SUFENTA, recovery times are more rapid compared to equipotent fentanyl dosages.
The vagolytic effects of pancuronium may produce a dose dependent elevation in heart rate during SUFENTA-oxygen anesthesia. The use of moderate doses of pancuronium or of a less vagolytic neuromuscular blocking agent may be used to maintain a stable lower heart rate and blood pressure during SUFENTA-oxygen anesthesia. The vagolytic effects of pancuronium may be reduced in patients administered nitrous oxide with SUFENTA.
Preliminary data suggest that in patients administered high doses of SUFENTA, initial dosage requirements for neuromuscular blocking agents are generally lower as compared to patients given fentanyl or halothane, and comparable to patients given enflurane.
Bradycardia is infrequently seen in patients administered SUFENTA-oxygen anesthesia. The use of nitrous oxide with high doses of SUFENTA may decrease mean arterial pressure, heart rate and cardiac output.
SUFENTA at 20 mcg/kg has been shown to provide more adequate reduction in intracranial volume than equivalent doses of fentanyl, based upon requirements for furosemide and anesthesia supplementation in one study of patients undergoing craniotomy. During carotid endarterectomy, SUFENTA-nitrous oxide/oxygen produced reductions in cerebral blood flow comparable to those of enflurane-nitrous oxide/oxygen. During cardiovascular surgery, SUFENTA-oxygen produced EEG patterns similar to fentanyl-oxygen; these EEG changes were judged to be compatible with adequate general anesthesia.
The intraoperative use of SUFENTA at anesthetic dosages maintains cardiac output, with a slight reduction in systemic vascular resistance during the initial postoperative period. The incidence of postoperative hypertension, need for vasoactive agents and requirements for postoperative analgesics are generally reduced in patients administered moderate or high doses of SUFENTA as compared to patients given inhalation agents.
Skeletal muscle rigidity is related to the dose and speed of administration of SUFENTA. This muscular rigidity may occur unless preventative measures are taken (see WARNINGS).
Decreased respiratory drive and increased airway resistance occur with SUFENTA. The duration and degree of respiratory depression are dose related when SUFENTA is used at sub-anesthetic dosages. At high doses, a pronounced decrease in pulmonary exchange and apnea may be produced.
Epidural use in Labor and Delivery
Onset of analgesic effect occurs within approximately 10 minutes of administration of epidural doses of SUFENTA and bupivacaine. Duration of analgesia following a single epidural injection of 10 to 15 mcg SUFENTA and bupivacaine 0.125% averaged 1.7 hours.
During labor and vaginal delivery, the addition of 10 to 15 mcg SUFENTA to 10 mL 0.125% bupivacaine provides an increase in the duration of analgesia compared to bupivacaine without an opioid. Analgesia from 15 mcg SUFENTA plus 10 mL 0.125% bupivacaine is comparable to analgesia from 10 mL of 0.25% bupivacaine alone. Apgar scores of neonates following epidural administration of both drugs to women in labor were comparable to neonates whose mothers received bupivacaine without an opioid epidurally.
Pharmacokinetics
Intravenous use
The pharmacokinetics of intravenous SUFENTA can be described as a three-compartment model, with a distribution time of 1.4 minutes, redistribution of 17.1 minutes and elimination half-life of 164 minutes in adults. The elimination half-life of SUFENTA is shorter (e.g. 97 +/- 42 minutes) in infants and children, and longer in neonates (e.g. 434 +/- 160 minutes) compared to that of adolescents and adults. The liver and small intestine are the major sites of biotransformation. Approximately 80% of the administered dose is excreted within 24 hours and only 2% of the dose is eliminated as unchanged drug. Plasma protein binding of sufentanil, related to the alpha acid glycoprotein concentration, was approximately 93% in healthy males, 91% in mothers and 79% in neonates.
Epidural use in Labor and Delivery
After epidural administration of incremental doses totaling 5 to 40 mcg SUFENTA during labor and delivery, maternal and neonatal sufentanil plasma concentrations were at or near the 0.05 to 0.1 ng/mL limit of detection, and were slightly higher in mothers than in their infants.
-
CLINICAL STUDIES
Epidural use in Labor and Delivery
Epidural sufentanil was tested in 340 patients in two (one single-center and one multicenter) double-blind, parallel studies. Doses ranged from 10 to 15 mcg sufentanil and were delivered in a 10 mL volume of 0.125% bupivacaine with and without epinephrine 1:200,000. In all cases sufentanil was administered following a dose of local anesthetic to test proper catheter placement. Since epidural opioids and local anesthetics potentiate each other, these results may not reflect the dose or efficacy of epidural sufentanil by itself.
Individual doses of 10 to 15 mcg SUFENTA plus bupivacaine 0.125% with epinephrine provided analgesia during the first stage of labor with a duration of 1 to 2 hours. Onset was rapid (within 10 minutes). Subsequent doses (equal dose) tended to have shorter duration. Analgesia was profound (complete pain relief) in 80% to 100% of patients and a 25% incidence of pruritus was observed. The duration of initial doses of SUFENTA plus bupivacaine with epinephrine is approximately 95 minutes, and of subsequent doses, 70 minutes.
There are insufficient data to critically evaluate neonatal neuromuscular and adaptive capacity following recommended doses of maternally administered epidural sufentanil with bupivacaine. However, if larger than recommended doses are used for combined local and systemic analgesia, e.g. after administration of a single dose of 50 mcg epidural sufentanil during delivery, then impaired neonatal adaption to sound and light can be detected for 1 to 4 hours and if a dose of 80 mcg is used impaired neuromuscular coordination can be detected for more than 4 hours.
-
INDICATIONS AND USAGE
SUFENTA (sufentanil citrate) is indicated for intravenous administration in adults and pediatric patients:
as an analgesic adjunct in the maintenance of balanced general anesthesia in patients who are intubated and ventilated.
as a primary anesthetic agent for the induction and maintenance of anesthesia with 100% oxygen in patients undergoing major surgical procedures, in patients who are intubated and ventilated, such as cardiovascular surgery or neurosurgical procedures in the sitting position, to provide favorable myocardial and cerebral oxygen balance or when extended postoperative ventilation is anticipated.
SUFENTA (sufentanil citrate) is indicated for epidural administration as an analgesic combined with low dose bupivacaine, usually 12.5 mg per administration, during labor and vaginal delivery.
SEE DOSAGE AND ADMINISTRATION SECTION FOR MORE COMPLETE INFORMATION ON THE USE OF SUFENTA.
- CONTRAINDICATIONS
-
WARNINGS
SUFENTA SHOULD BE ADMINISTERED ONLY BY PERSONS SPECIFICALLY TRAINED IN THE USE OF INTRAVENOUS AND EPIDURAL ANESTHETICS AND MANAGEMENT OF THE RESPIRATORY EFFECTS OF POTENT OPIOIDS.
AN OPIOID ANTAGONIST, RESUSCITATIVE AND INTUBATION EQUIPMENT AND OXYGEN SHOULD BE READILY AVAILABLE.
PRIOR TO CATHETER INSERTION, THE PHYSICIAN SHOULD BE FAMILIAR WITH PATIENT CONDITIONS (SUCH AS INFECTION AT THE INJECTION SITE, BLEEDING DIATHESIS, ANTICOAGULANT THERAPY, ETC.) WHICH CALL FOR SPECIAL EVALUATION OF THE BENEFIT VERSUS RISK POTENTIAL.
Intravenous use
Intravenous administration or unintentional intravascular injection during epidural administration of SUFENTA may cause skeletal muscle rigidity, particularly of the truncal muscles. The incidence and severity of muscle rigidity is dose related. Administration of SUFENTA may produce muscular rigidity with a more rapid onset of action than that seen with fentanyl. SUFENTA may produce muscular rigidity that involves the skeletal muscles of the neck and extremities. As with fentanyl, muscular rigidity has been reported to occur or recur infrequently in the extended postoperative period. The incidence of muscular rigidity associated with intravenous SUFENTA can be reduced by: 1) administration of up to 1/4 of the full paralyzing dose of a non-depolarizing neuromuscular blocking agent just prior to administration of SUFENTA at dosages of up to 8 mcg/kg, 2) administration of a full paralyzing dose of a neuromuscular blocking agent following loss of consciousness when SUFENTA is used in anesthetic dosages (above 8 mcg/kg) titrated by slow intravenous infusion, or, 3) simultaneous administration of SUFENTA and a full paralyzing dose of a neuromuscular blocking agent when SUFENTA is used in rapidly administered anesthetic dosages (above 8 mcg/kg).
The neuromuscular blocking agents used should be compatible with the patient's cardiovascular status. Adequate facilities should be available for postoperative monitoring and ventilation of patients administered SUFENTA. It is essential that these facilities be fully equipped to handle all degrees of respiratory depression.
-
PRECAUTIONS
General
The initial dose of SUFENTA should be appropriately reduced in elderly and debilitated patients. The effect of the initial dose should be considered in determining supplemental doses.
Vital signs should be monitored routinely.
Nitrous oxide may produce cardiovascular depression when given with high doses of SUFENTA (see CLINICAL PHARMACOLOGY).
Bradycardia has been reported infrequently with SUFENTA-oxygen anesthesia and has been responsive to atropine.
Respiratory depression caused by opioid analgesics can be reversed by opioid antagonists such as naloxone. Because the duration of respiratory depression produced by SUFENTA may last longer than the duration of the opioid antagonist action, appropriate surveillance should be maintained. As with all potent opioids, profound analgesia is accompanied by respiratory depression and diminished sensitivity to CO2 stimulation which may persist into or recur in the postoperative period. Respiratory depression may be enhanced when SUFENTA is administered in combination with volatile inhalational agents and/or other central nervous system depressants such as barbiturates, tranquilizers, and other opioids. Appropriate postoperative monitoring should be employed to ensure that adequate spontaneous breathing is established and maintained prior to discharging the patient from the recovery area. Respiration should be closely monitored following each administration of an epidural injection of SUFENTA.
Proper placement of the needle or catheter in the epidural space should be verified before SUFENTA is injected to assure that unintentional intravascular or intrathecal administration does not occur. Unintentional intravascular injection of SUFENTA could result in a potentially serious overdose, including acute truncal muscular rigidity and apnea. Unintentional intrathecal injection of the full sufentanil/bupivacaine epidural doses and volume could produce effects of high spinal anesthesia including prolonged paralysis and delayed recovery. If analgesia is inadequate, the placement and integrity of the catheter should be verified prior to the administration of any additional epidural medications. SUFENTA should be administered epidurally by slow injection.
Neuromuscular Blocking Agents
The hemodynamic effects and degree of skeletal muscle relaxation required should be considered in the selection of a neuromuscular blocking agent. High doses of pancuronium may produce increases in heart rate during SUFENTA-oxygen anesthesia. Bradycardia and hypotension have been reported with other muscle relaxants during SUFENTA-oxygen anesthesia; this effect may be more pronounced in the presence of calcium channel and/or beta-blockers. Muscle relaxants with no clinically significant effect on heart rate (at recommended doses) would not counteract the vagotonic effect of SUFENTA, therefore a lower heart rate would be expected. Rare reports of bradycardia associated with the concomitant use of succinylcholine and SUFENTA have been reported.
Interaction with Calcium Channel and Beta Blockers
The incidence and degree of bradycardia and hypotension during induction with SUFENTA may be greater in patients on chronic calcium channel and beta blocker therapy. (See Neuromuscular Blocking Agents.)
Interaction with Other Central Nervous System Depressants
Both the magnitude and duration of central nervous system and cardiovascular effects may be enhanced when SUFENTA is administered to patients receiving barbiturates, tranquilizers, other opioids, general anesthetic or other CNS depressants. In such cases of combined treatment, the dose of SUFENTA and/or these agents should be reduced.
The use of benzodiazepines with SUFENTA during induction may result in a decrease in mean arterial pressure and systemic vascular resistance.
Impaired Respiration
SUFENTA should be used with caution in patients with pulmonary disease, decreased respiratory reserve or potentially compromised respiration. In such patients, opioids may additionally decrease respiratory drive and increase airway resistance. During anesthesia, this can be managed by assisted or controlled respiration.
Carcinogenesis, Mutagenesis and Impairment of Fertility
No long-term animal studies of SUFENTA have been performed to evaluate carcinogenic potential. The micronucleus test in female rats revealed that single intravenous doses of SUFENTA as high as 80 mcg/kg (approximately 2.5 times the upper human intravenous dose) produced no structural chromosome mutations. The Ames Salmonella typhimurium metabolic activating test also revealed no mutagenic activity. See ANIMAL TOXICOLOGY for reproduction studies in rats and rabbits.
Pregnancy Category C
SUFENTA has been shown to have an embryocidal effect in rats and rabbits when given in doses 2.5 times the upper human intravenous dose for a period of 10 days to over 30 days. These effects were most probably due to maternal toxicity (decreased food consumption with increased mortality) following prolonged administration of the drug.
No evidence of teratogenic effects have been observed after administration of SUFENTA in rats or rabbits.
Labor and Delivery
The use of epidurally administered SUFENTA in combination with bupivacaine 0.125% with or without epinephrine is indicated for labor and delivery. (See INDICATIONS AND USAGE and DOSAGE AND ADMINISTRATION sections.) SUFENTA is not recommended for intravenous use or for use of larger epidural doses during labor and delivery because of potential risks to the newborn infant after delivery. In clinical trials, one case of severe fetal bradycardia associated with maternal hypotension was reported within 8 minutes of maternal administration of sufentanil 15 mcg plus bupivacaine 0.125% (10 mL total volume).
Nursing Mothers
It is not known whether sufentanil is excreted in human milk. Because fentanyl analogs are excreted in human milk, caution should be exercised when SUFENTA is administered to a nursing woman.
Pediatric Use
The safety and efficacy of intravenous SUFENTA in pediatric patients as young as 1 day old undergoing cardiovascular surgery have been documented in a limited number of cases. The clearance of SUFENTA in healthy neonates is approximately one-half that in adults and children. The clearance rate of SUFENTA can be further reduced by up to a third in neonates with cardiovascular disease, resulting in an increase in the elimination half-life of the drug.
Animal Toxicology
The intravenous LD50 of SUFENTA is 16.8 to 18.0 mg/kg in mice, 11.8 to 13.0 mg/kg in guinea pigs and 10.1 to 19.5 mg/kg in dogs. Reproduction studies performed in rats and rabbits given doses of up to 2.5 times the upper human intravenous dose for a period of 10 to over 30 days revealed high maternal mortality rates due to decreased food consumption and anoxia, which preclude any meaningful interpretation of the results. Epidural and intrathecal injections of sufentanil in dogs and epidural injections in rats were not associated with neurotoxicity.
-
ADVERSE REACTIONS
The most common adverse reactions of opioids are respiratory depression and skeletal muscle rigidity, particularly of the truncal muscles. SUFENTA may produce muscular rigidity that involves the skeletal muscles of the neck and extremities. See CLINICAL PHARMACOLOGY, WARNINGS and PRECAUTIONS on the management of respiratory depression and skeletal muscle rigidity. Urinary retention has been associated with the use of epidural opioids but was not reported in the clinical trials of epidurally administered sufentanil due to the use of indwelling catheters. The incidence of urinary retention in patients without urinary catheters receiving epidural sufentanil is unknown; return of normal bladder activity may be delayed.
The following adverse reaction information is derived from controlled clinical trials in 320 patients who received intravenous sufentanil during surgical anesthesia and in 340 patients who received epidural sufentanil plus bupivacaine 0.125% for analgesia during labor and is presented below. Based on the observed frequency, none of the reactions occurring with an incidence less than 1% were observed during clinical trials of epidural sufentanil used during labor and delivery (N=340).
In general cardiovascular and musculoskeletal adverse experiences were not observed in clinical trials of epidural sufentanil. Hypotension was observed 7 times more frequently in intravenous trials than in epidural trials. The incidence of central nervous system, dermatological and gastrointestinal adverse experiences was approximately 4 to 25 times higher in studies of epidural use in labor and delivery.
Probably Causally Related: Incidence Greater than 1% - Derived from clinical trials (See preceding paragraph)
Cardiovascular: bradycardia1, hypertension1, hypotension1.
Musculoskeletal: chest wall rigidity1.
Central Nervous System: somnolence1.
Dermatological: pruritus (25%).
Gastrointestinal: nausea1, vomiting1.
Probably Causally Related: Incidence Less than 1% - Derived from clinical trials (Adverse events reported in post-marketing surveillance, not seen in clinical trials, are italicized.)
Body as a whole: anaphylaxis.
Cardiovascular: arrhythmia2, tachycardia2, cardiac arrest.
Central Nervous System: chills2.
Dermatological: erythema2.
Musculoskeletal: skeletal muscle rigidity of neck and extremities.
Respiratory: apnea2, bronchospasm2, postoperative respiratory depression2.
Miscellaneous: intraoperative muscle movement2.
- DRUG ABUSE AND DEPENDENCE
-
OVERDOSAGE
Overdosage is manifested by an extension of the pharmacological actions of SUFENTA (see CLINICAL PHARMACOLOGY) as with other potent opioid analgesics. The most serious and significant effect of overdose for both intravenous and epidural administration of SUFENTA is respiratory depression. Intravenous administration of an opioid antagonist such as naloxone should be employed as a specific antidote to manage respiratory depression. The duration of respiratory depression following overdosage with SUFENTA may be longer than the duration of action of the opioid antagonist. Administration of an opioid antagonist should not preclude more immediate countermeasures. In the event of overdosage, oxygen should be administered and ventilation assisted or controlled as indicated for hypoventilation or apnea. A patent airway must be maintained, and a nasopharyngeal airway or endotracheal tube may be indicated. If depressed respiration is associated with muscular rigidity, a neuromuscular blocking agent may be required to facilitate assisted or controlled respiration. Intravenous fluids and vasopressors for the treatment of hypotension and other supportive measures may be employed.
-
DOSAGE AND ADMINISTRATION
The dosage of SUFENTA should be individualized in each case according to body weight, physical status, underlying pathological condition, use of other drugs, and type of surgical procedure and anesthesia. In obese patients (more than 20% above ideal total body weight), the dosage of SUFENTA should be determined on the basis of lean body weight. Dosage should be reduced in elderly and debilitated patients (see PRECAUTIONS).
Vital signs should be monitored routinely.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Because the clearance of SUFENTA is reduced in neonates, especially those with cardiovascular disease, the dose of SUFENTA should be reduced accordingly (see PRECAUTIONS).
Intravenous use
SUFENTA may be administered intravenously by slow injection or infusion 1) in doses of up to 8 mcg/kg as an analgesic adjunct to general anesthesia, and 2) in doses ≥8 mcg/kg as a primary anesthetic agent for induction and maintenance of anesthesia (see Dosage Range Chart). If benzodiazepines, barbiturates, inhalation agents, other opioids or other central nervous system depressants are used concomitantly, the dose of SUFENTA and/or these agents should be reduced (see PRECAUTIONS). In all cases dosage should be titrated to individual patient response.
Usage in Children
For induction and maintenance of anesthesia in children less than 12 years of age undergoing cardiovascular surgery, an anesthetic dose of 10 to 25 mcg/kg administered with 100% oxygen is generally recommended. Supplemental dosages of up to 25 to 50 mcg are recommended for maintenance, based on response to initial dose and as determined by changes in vital signs indicating surgical stress or lightening of anesthesia.
Premedication
The selection of preanesthetic medications should be based upon the needs of the individual patient.
Neuromuscular Blocking Agents
The neuromuscular blocking agent selected should be compatible with the patient's condition, taking into account the hemodynamic effects of a particular muscle relaxant and the degree of skeletal muscle relaxation required (see CLINICAL PHARMACOLOGY, WARNINGS and PRECAUTIONS).
ADULT DOSAGE RANGE CHART for Intravenous use ANALGESIC COMPONENT TO GENERAL ANESTHESIA
•TOTAL DOSAGE REQUIREMENTS OF 1 MCG/KG/HR OR LESS ARE RECOMMENDEDTOTAL DOSAGE MAINTENANCE DOSAGE ANALGESIC DOSAGES Incremental or Infusion: 1 to 2 mcg/kg (expected duration of anesthesia 1 to 2 hours). Approximately 75% or more of total SUFENTA dosage may be administered prior to intubation by either slow injection or infusion titrated to individual patient response. Dosages in this range are generally administered with nitrous oxide/oxygen in patients undergoing general surgery in which endotracheal intubation and mechanical ventilation are required. Incremental: 10 to 25 mcg (0.2 to 0.5 mL) may be administered in increments as needed when movement and/or changes in vital signs indicate surgical stress or lightening of analgesia. Supplemental dosages should be individualized and adjusted to remaining operative time anticipated.
Infusion: SUFENTA may be administered as an intermittent or continuous infusion as needed in response to signs of lightening of analgesia. In absence of signs of lightening of analgesia, infusion rates should always be adjusted downward until there is some response to surgical stimulation. Maintenance infusion rates should be adjusted based upon the induction dose of SUFENTA so that the total dose does not exceed 1 mcg/kg/hr of expected surgical time. Dosage should be individualized and adjusted to remaining operative time anticipated.ANALGESIC DOSAGES Incremental or Infusion: 2 to 8 mcg/kg (expected duration of anesthesia 2 to 8 hours). Approximately 75% or less of the total calculated SUFENTA dosage may be administered by slow injection or infusion prior to intubation, titrated to individual patient response. Dosages in this range are generally administered with nitrous oxide/oxygen in patients undergoing more complicated major surgical procedures in which endotracheal intubation and mechanical ventilation are required. At dosages in this range, SUFENTA has been shown to provide some attenuation of sympathetic reflex activity in response to surgical stimuli, provide hemodynamic stability, and provide relatively rapid recovery. Incremental: 10 to 50 mcg (0.2 to 1 mL) may be administered in increments as needed when movement and/or changes in vital signs indicate surgical stress or lightening of analgesia. Supplemental dosages should be individualized and adjusted to the remaining operative time anticipated.
Infusion: SUFENTA may be administered as an intermittent or continuous infusion as needed in response to signs of lightening of analgesia. In the absence of signs of lightening of analgesia, infusion rates should always be adjusted downward until there is some response to surgical stimulation. Maintenance infusion rates should be adjusted based upon the induction dose of SUFENTA so that the total dose does not exceed 1 mcg/kg/hr of expected surgical time. Dosage should be individualized and adjusted to remaining operative time anticipated.ANESTHETIC DOSAGES Incremental or Infusion: 8 to 30 mcg/kg (anesthetic doses). At this anesthetic dosage range SUFENTA is generally administered as a slow injection, as an infusion, or as an injection followed by an infusion. SUFENTA with 100% oxygen and a muscle relaxant has been found to produce sleep at dosages ≥8 mcg/kg and to maintain a deep level of anesthesia without the use of additional anesthetic agents. The addition of N2O to these dosages will reduce systolic blood pressure. At dosages in this range of up to 25 mcg/kg, catecholamine release is attenuated. Dosages of 25 to 30 mcg/kg have been shown to block sympathetic response including catecholamine release. High doses are indicated in patients undergoing major surgical procedures, in which endotracheal intubation and mechanical ventilation are required, such as cardiovascular surgery and neurosurgery in the sitting position with maintenance of favorable myocardial and cerebral oxygen balance. Postoperative observation is essential and postoperative mechanical ventilation may be required at the higher dosage range due to extended postoperative respiratory depression. Dosage should be titrated to individual patient response. Incremental: Depending on the initial dose, maintenance doses of 0.5 to 10 mcg/kg may be administered by slow injection in anticipation of surgical stress such as incision, sternotomy or cardiopulmonary bypass.
Infusion: SUFENTA may be administered by continuous or intermittent infusion as needed in response to signs of lightening of anesthesia. In the absence of lightening of anesthesia, infusion rates should always be adjusted downward until there is some response to surgical stimulation. The maintenance infusion rate for SUFENTA should be based upon the induction dose so that the total dose for the procedure does not exceed 30 mcg/kg.In patients administered high doses of SUFENTA, it is essential that qualified personnel and adequate facilities are available for the management of postoperative respiratory depression.
Also see WARNINGS and PRECAUTIONS sections.
For purposes of administering small volumes of SUFENTA accurately, the use of a tuberculin syringe or equivalent is recommended.
Epidural use in Labor and Delivery Proper placement of the needle or catheter in the epidural space should be verified before SUFENTA is injected to assure that unintentional intravascular or intrathecal administration does not occur. Unintentional intravascular injection of SUFENTA could result in a potentially serious overdose, including acute truncal muscular rigidity and apnea. Unintentional intrathecal injection of the full sufentanil, bupivacaine epidural doses and volume could produce effects of high spinal anesthesia including prolonged paralysis and delayed recovery. If analgesia is inadequate, the placement and integrity of the catheter should be verified prior to the administration of any additional epidural medications. SUFENTA should be administered by slow injection. Respiration should be closely monitored following each administration of an epidural injection of SUFENTA. Dosage for Labor and Delivery: The recommended dosage is SUFENTA 10 to 15 mcg administered with 10 mL bupivacaine 0.125% with or without epinephrine. SUFENTA and bupivacaine should be mixed together before administration. Doses can be repeated twice (for a total of three doses) at not less than one-hour intervals until delivery. -
HOW SUPPLIED
SUFENTA (Sufentanil Citrate Injection, USP) is supplied as a sterile aqueous preservative-free solution for intravenous and epidural use as:
NDC 11098-050-01 50 mcg/mL sufentanil base, 1 mL ampules in packages of 10
NDC 11098-050-02 50 mcg/mL sufentanil base, 2 mL ampules in packages of 10
NDC 11098-050-05 50 mcg/mL sufentanil base, 5 mL ampules in packages of 10 - SPL UNCLASSIFIED SECTION
-
INGREDIENTS AND APPEARANCE
SUFENTA
sufentanil citrate solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:11098-050 Route of Administration INTRAVENOUS DEA Schedule CII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength sufentanil citrate (UNII: S9ZFX8403R) (sufentanil - UNII:AFE2YW0IIZ) 50 ug in 1 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11098-050-01 10 in 1 CARTON 1 1 mL in 1 AMPULE 2 NDC:11098-050-02 10 in 1 CARTON 2 2 mL in 1 AMPULE 3 NDC:11098-050-05 10 in 1 CARTON 3 5 mL in 1 AMPULE Labeler - Taylor Pharmaceuticals
