Label: NYSTATIN suspension
-
Contains inactivated NDC Code(s)
NDC Code(s): 17856-0811-1, 17856-0811-2, 17856-0811-3, 17856-0811-4, view more17856-0811-5, 17856-0811-6, 17856-0811-7 - Packager: ATLANTIC BIOLOGICALS CORP.
- This is a repackaged label.
- Source NDC Code(s): 0121-0810
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 26, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Nystatin is an antimycotic polyene antibiotic obtained from Streptomyces noursei. Structural formula:
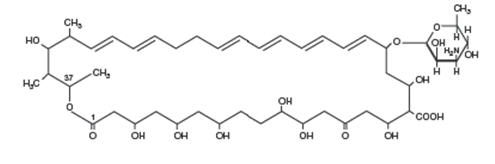
C 47H 75NO 17 MW 926.13
Nystatin Oral Suspension USP, for oral administration, contains 100,000 USP Nystatin Units per mL. Inactive ingredients: alcohol (≤ 1% v/v), artificial peppermint flavor, cherry flavor, citric acid, D&C Yellow No. 10, FD&C Red No. 40, glycerin, magnesium aluminum silicate, methylparaben, potassium phosphate dibasic, propylene glycol, propylparaben, purified water and sucrose.
-
CLINICAL PHARMACOLOGY
Pharmacokinetics
Gastrointestinal absorption of nystatin is insignificant. Most orally administered nystatin is passed unchanged in the stool. In patients with renal insufficiency receiving oral therapy with conventional dosage forms, significant plasma concentrations of nystatin may occasionally occur.
Microbiology
Nystatin is both fungistatic and fungicidal in vitro against a wide variety of yeasts and yeast-like fungi. Candida albicans demonstrates no significant resistance to nystatin in vitro on repeated subculture in increasing levels of nystatin; other Candida species become quite resistant. Generally, resistance does not develop in vivo. Nystatin acts by binding to sterols in the cell membrane of susceptible Candida species with a resultant change in membrane permeability allowing leakage of intracellular components. Nystatin exhibits no appreciable activity against bacteria, protozoa, or viruses.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
PRECAUTIONS
General
This medication is not to be used for the treatment of systemic mycoses. Discontinue treatment if sensitization or irritation is reported during use.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term animal studies have been performed to evaluate carcinogenic potential. There also have been no studies to determine mutagenicity or whether this medication affects fertility in males or females.
Pregnancy
Teratogenic Effects Category C
Animal reproduction studies have not been conducted with nystatin oral suspension. It is also not known whether nystatin oral suspension can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Nystatin oral suspension should be given to a pregnant woman only if clearly needed.
-
ADVERSE REACTIONS
Nystatin is well tolerated even with prolonged therapy. Oral irritation and sensitization have been reported. (See PRECAUTIONS: General).
Gastrointestinal: Diarrhea (including one case of bloody diarrhea), nausea, vomiting, gastrointestinal upset/disturbances.
Dermatologic: Rash, including urticaria has been reported rarely. Stevens-Johnson syndrome has been reported very rarely.
Other: Tachycardia, bronchospasm, facial swelling, and non-specific myalgia have also been rarely reported.
-
OVERDOSAGE
Oral doses of nystatin in excess of five million units daily have caused nausea and gastrointestinal upset. There have been no reports of serious toxic effects of superinfections (See CLINICAL PHARMACOLOGY, Pharmacokinetics).
-
DOSAGE AND ADMINISTRATION
INFANTS: 2 mL (200,000 units) four times daily (in infants and young children, use dropper to place one-half of dose in each side of mouth and avoid feeding for 5 to 10 minutes).
NOTE: Limited clinical studies in premature and low birth weight infants indicate that 1 mL four times daily is effective.
CHILDREN AND ADULTS: 4 to 6 mL (400,000 to 600,000 units) four times daily (one-half of dose in each side of mouth). The preparation should be retained in the mouth as long as possible before swallowing.
Continue treatment for at least 48 hours after perioral symptoms have disappeared and cultures demonstrate eradication of Candida albicans.
-
HOW SUPPLIED
Nystatin Oral Suspension USP, 100,000 USP Nystatin Units per mL, is available in a cherry, peppermint flavored, light creamy yellow, ready-to-use suspension, supplied in the following oral dosage forms:
NDC 17856-0811-01 NYSTATIN 100,000 UNITS/ML 0.5ML ENFIT SYRINGE 120 ct UD
NDC 17856-0811-02 NYSTATIN 100,000 UNITS/ML 1ML ENFIT SYRINGE 120 ct UD
NDC 17856-0811-03 NYSTATIN 100,000 UNITS/ML 2ML ENFIT SYRINGE 120 ct UD
NDC 17856-0811-04 NYSTATIN 100,000 UNITS/ML 5ML ENFIT SYRINGE 48 ct UD
NDC 17856-0811-05 NYSTATIN 100,000 UNITS/ML 5ML CUP 72 ct UD
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NYSTATIN
nystatin suspensionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:17856-0811(NDC:0121-0810) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NYSTATIN (UNII: BDF1O1C72E) (NYSTATIN - UNII:BDF1O1C72E) NYSTATIN 100000 [USP'U] in 1 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C RED NO. 40 (UNII: WZB9127XOA) GLYCERIN (UNII: PDC6A3C0OX) METHYLPARABEN (UNII: A2I8C7HI9T) POTASSIUM PHOSPHATE, DIBASIC (UNII: CI71S98N1Z) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) SUCROSE (UNII: C151H8M554) MAGNESIUM ALUMINUM SILICATE (UNII: 6M3P64V0NC) Product Characteristics Color yellow (Light - Creamy) Score Shape Size Flavor CHERRY (w/Peppermint) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17856-0811-1 120 in 1 BOX, UNIT-DOSE 01/26/2021 1 0.5 mL in 1 SYRINGE; Type 0: Not a Combination Product 2 NDC:17856-0811-2 120 in 1 BOX, UNIT-DOSE 01/26/2021 2 1 mL in 1 SYRINGE; Type 0: Not a Combination Product 3 NDC:17856-0811-3 120 in 1 BOX, UNIT-DOSE 01/26/2021 3 2 mL in 1 SYRINGE; Type 0: Not a Combination Product 4 NDC:17856-0811-4 48 in 1 BOX, UNIT-DOSE 01/26/2021 4 5 mL in 1 SYRINGE; Type 0: Not a Combination Product 5 NDC:17856-0811-5 72 in 1 BOX, UNIT-DOSE 01/26/2021 5 5 mL in 1 CUP, UNIT-DOSE; Type 0: Not a Combination Product 6 NDC:17856-0811-6 120 in 1 BOX, UNIT-DOSE 01/26/2021 6 1 mL in 1 SYRINGE; Type 0: Not a Combination Product 7 NDC:17856-0811-7 120 in 1 BOX, UNIT-DOSE 01/26/2021 7 2 mL in 1 SYRINGE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA203621 05/07/2019 Labeler - ATLANTIC BIOLOGICALS CORP. (047437707) Establishment Name Address ID/FEI Business Operations ATLANTIC BIOLOGICALS CORP. 047437707 relabel(17856-0811) , repack(17856-0811)










