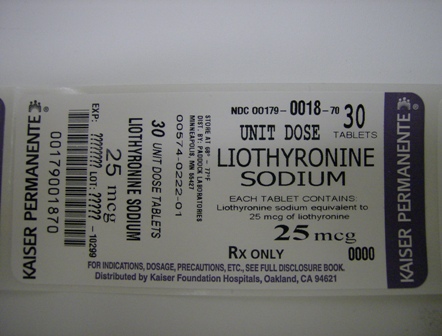
LIOTHYRONINE SODIUM- liothyronine sodium tablet
KAISER FOUNDATION HOSPITALS
----------
Thyroid hormone drugs are natural or synthetic preparations containing tetraiodothyronine (T4, levothyroxine) sodium or triiodothyronine (T3, liothyronine) sodium or both. T4 and T3 are produced in the human thyroid gland by the iodination and coupling of the amino acid tyrosine. T4 contains four iodine atoms and is formed by the coupling of two molecules of diiodotyrosine (DIT). T3 contains three atoms of iodine and is formed by the coupling of one molecule of DIT with one molecule of monoiodotyrosine (MIT). Both hormones are stored in the thyroid colloid as thyroglobulin.
Thyroid hormone preparations belong to two categories: (1) natural hormonal preparations derived from animal thyroid, and (2) synthetic preparations. Natural preparations include desiccated thyroid and thyroglobulin. Desiccated thyroid is derived from domesticated animals that are used for food by man (either beef or hog thyroid), and thyroglobulin is derived from thyroid glands of the hog. The United States Pharmacopeia (USP) has standardized the total iodine content of natural preparations. Thyroid USP contains not less than (NLT) 0.17 percent and not more than (NMT) 0.23 percent iodine, and thyroglobulin contains not less than (NLT) 0.7 percent of organically bound iodine. Iodine content is only an indirect indicator of true hormonal biologic activity.
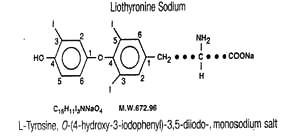
Liothyronine Sodium Tablets, USP contain liothyronine (L-triiodothyronine or LT3), a synthetic form of a natural thyroid hormone, and is available as the sodium salt.
The structural and empirical formulas and molecular weight of liothyronine sodium are given below.
Twenty-five mcg of liothyronine is equivalent to approximately 1 grain of desiccated thyroid or thyroglobulin and 0.1 mg of L-thyroxine.
Each round, white Liothyronine Sodium Tablet, USP contains liothyronine sodium equivalent to liothyronine as follows: 5 mcg debossed 5 and 220; 25 mcg scored and debossed 25 and 222; 50 mcg scored and debossed 50 and 223. Inactive ingredients consist of calcium sulfate, microcrystalline cellulose, hypromellose, talc, and colloidal silicon dioxide.
CLINICAL PHARMACOLOGY
The mechanisms by which thyroid hormones exert their physiologic action are not well understood. These hormones enhance oxygen consumption by most tissues of the body, increase the basal metabolic rate and the metabolism of carbohydrates, lipids and proteins. Thus, they exert a profound influence on every organ system in the body and are of particular importance in the development of the central nervous system.
Pharmacokinetics
Since liothyronine sodium (T3) is not firmly bound to serum protein, it is readily available to body tissues. The onset of activity of liothyronine sodium is rapid, occurring within a few hours. Maximum pharmacologic response occurs within 2 or 3 days, providing early clinical response. The biological half-life is about 2-1/2 days.
T3 is almost totally absorbed, 95 percent in 4 hours. The hormones contained in the natural preparations are absorbed in a manner similar to the synthetic hormones.
Liothyronine sodium has a rapid cutoff of activity which permits quick dosage adjustment and facilitates control of the effects of overdosage, should they occur.
The higher affinity of levothyroxine (T4) for both thyroid-binding globulin and thyroid-binding prealbumin as compared to triiodothyronine (T3) partially explains the higher serum levels and longer half-life of the former hormone. Both protein-bound hormones exist in reverse equilibrium with minute amounts of free hormone, the latter accounting for the metabolic activity.
INDICATIONS AND USAGE
Thyroid hormone drugs are indicated:
1. As replacement or supplemental therapy in patients with hypothyroidism of any etiology, except transient hypothyroidism during the recovery phase of subacute thyroiditis. This category includes cretinism, myxedema and ordinary hypothyroidism in patients of any age (pediatric patients, adults, the elderly), or state (including pregnancy); primary hypothyroidism resulting from functional deficiency, primary atrophy, partial or total absence of thyroid gland, or the effects of surgery, radiation, or drugs, with or without the presence of goiter; and secondary (pituitary) or tertiary (hypothalamic) hypothyroidism (see WARNINGS).
2. As pituitary thyroid-stimulating hormone (TSH) suppressants, in the treatment or prevention of various types of euthyroid goiters, including thyroid nodules, subacute or chronic lymphocytic thyroiditis (Hashimoto's) and multinodular goiter.
3. As diagnostic agents in suppression tests to differentiate suspected mild hyperthyroidism or thyroid gland autonomy.
Liothyronine Sodium Tablets, USP can be used in patients allergic to desiccated thyroid or thyroid extract derived from pork or beef.
CONTRAINDICATIONS
Thyroid hormone preparations are generally contraindicated in patients with diagnosed but as yet uncorrected adrenal cortical insufficiency, untreated thyrotoxicosis and apparent hypersensitivity to any of their active or extraneous constituents. There is no well-documented evidence from the literature, however, of true allergic or idiosyncratic reactions to thyroid hormone.
ADVERSE REACTIONS
Adverse reactions, other than those indicative of hyperthyroidism because of therapeutic overdosage, either initially or during the maintenance period are rare (see OVERDOSAGE).
In rare instances, allergic skin reactions have been reported with Liothyronine Sodium Tablets, USP.
DOSAGE AND ADMINISTRATION
The dosage of thyroid hormones is determined by the indication and must in every case be individualized according to patient response and laboratory findings.
Liothyronine Sodium Tablets, USP are intended for oral administration; once-a-day dosage is recommended. Although liothyronine sodium has a rapid cutoff, its metabolic effects persist for a few days following discontinuance.
Mild Hypothyroidism
Recommended starting dosage is 25 mcg daily. Daily dosage then may be increased by up to 25 mcg every 1 or 2 weeks. Usual maintenance dose is 25 to 75 mcg daily.
The rapid onset and dissipation of action of liothyronine sodium (T3), as compared with levothyroxine sodium (T4), has led some clinicians to prefer its use in patients who might be more susceptible to the untoward effects of thyroid medication. However, the wide swings in serum T3 levels that follow its administration and the possibility of more pronounced cardiovascular side effects tend to counterbalance the stated advantages.
Liothyronine Sodium Tablets, USP may be used in preference to levothyroxine (T4) during radioisotope scanning procedures, since induction of hypothyroidism in those cases is more abrupt and can be of shorter duration. It may also be preferred when impairment of peripheral conversion of T4 to T3 is suspected.
Myxedema Coma
Myxedema coma is usually precipitated in the hypothyroid patient of long standing by intercurrent illness or drugs such as sedatives and anesthetics and should be considered a medical emergency.
An intravenous preparation of liothyronine sodium is marketed under the trade name Triostat® for use in myxedema coma/precoma.
Congenital Hypothyroidism
Recommended starting dosage is 5 mcg daily, with a 5 mcg increment every 3 to 4 days until the desired response is achieved. Infants a few months old may require only 20 mcg daily for maintenance. At 1 year, 50 mcg daily may be required. Above 3 years, full adult dosage may be necessary (see PRECAUTIONS, Pediatric Use).
Simple (non-toxic) Goiter
Recommended starting dosage is 5 mcg daily. This dosage may be increased by 5 to 10 mcg daily every 1 or 2 weeks. When 25 mcg daily is reached, dosage may be increased every week or two by 12.5 or 25 mcg. Usual maintenance dosage is 75 mcg daily.
In the elderly or in pediatric patients, therapy should be started with 5 mcg daily and increased only by 5 mcg increments at the recommended intervals.
When switching a patient to Liothyronine Sodium Tablets, USP from thyroid, L-thyroxine or thyroglobulin, discontinue the other medication, initiate Liothyronine Sodium Tablets, USP at a low dosage, and increase gradually according to the patient's response. When selecting a starting dosage, bear in mind that this drug has a rapid onset of action, and that residual effects of the other thyroid preparation may persist for the first several weeks of therapy.
Thyroid Suppression Therapy
Administration of thyroid hormone in doses higher than those produced physiologically by the gland results in suppression of the production of endogenous hormone. This is the basis for the thyroid suppression test and is used as an aid in the diagnosis of patients with signs of mild hyperthyroidism in whom baseline laboratory tests appear normal or to demonstrate thyroid gland autonomy in patients with Graves' ophthalmopathy. 131I uptake is determined before and after the administration of the exogenous hormone. A 50% or greater suppression of uptake indicates a normal thyroid-pituitary axis and thus rules out thyroid gland autonomy.
Liothyronine Sodium Tablets, USP are given in doses of 75 to 100 mcg/day for 7 days, and radioactive iodine uptake is determined before and after administration of the hormone. If thyroid function is under normal control, the radioiodine uptake will drop significantly after treatment. Liothyronine Sodium Tablets, USP should be administered cautiously to patients in whom there is a strong suspicion of thyroid gland autonomy, in view of the fact that the exogenous hormone effects will be additive to the endogenous source.
HOW SUPPLIED
Liothyronine Sodium Tablets, USP, 25 mcg, are white to off-white, round, flat, debossed "25" above the score and "222" below the score on one side and plain on the other.
NDC 0179-0018-70 30 tablets in 1 Box, UNIT-DOSE
| LIOTHYRONINE SODIUM
liothyronine sodium tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - KAISER FOUNDATION HOSPITALS (053052619) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| KAISER FOUNDATION HOSPITALS | 053052619 | repack(0179-0018) | |