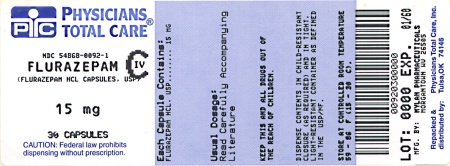
Label: FLURAZEPAM HYDROCHLORIDE capsule
-
Contains inactivated NDC Code(s)
NDC Code(s): 54868-0092-1, 54868-0092-2, 54868-0093-0, 54868-0093-1 - Packager: Physicians Total Care, Inc.
- This is a repackaged label.
- Source NDC Code(s): 0378-4415, 0378-4430
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CIV
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 3, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
DESCRIPTION
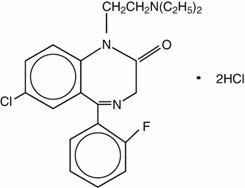
Flurazepam hydrochloride is chemically 7-chloro-1-[2-(diethylamino)ethyl]-5-(o-fluoro-phenyl)-1,3dihydro-2H-1,4-benzodiazepin-2-one dihydrochloride. It is a pale yellow, crystalline compound, freely soluble in alcohol and very soluble in water. It has a molecular weight of 460.81 and the following structural formula:
Each capsule for oral administration contains either 15 mg or 30 mg of flurazepam hydrochloride, USP and the following inactive ingredients: colloidal silicon dioxide, magnesium stearate, microcrystalline cellulose, powdered cellulose and sodium lauryl sulfate. The empty gelatin capsule contains FD&C Blue No. 1, FD&C Red No. 3, gelatin and titanium dioxide.
The imprinting ink contains black iron oxide, D&C Yellow No. 10 Aluminum Lake, FD&C Blue No. 1 Aluminum Lake, FD&C Blue No. 2 Aluminum Lake, FD&C Red No. 40 Aluminum Lake, propylene glycol and shellac glaze.
-
CLINICAL PHARMACOLOGY
Flurazepam hydrochloride is rapidly absorbed from the G.I. tract. Flurazepam is rapidly metabolized and is excreted primarily in the urine. Following a single oral dose, peak flurazepam plasma concentrations ranging from 0.5 to 4.0 ng/mL occur at 30 to 60 minutes post-dosing. The harmonic mean apparent half-life of flurazepam is 2.3 hours. The blood level profile of flurazepam and its major metabolites was determined in man following the oral administration of 30 mg daily for 2 weeks. The N1-hydroxyethyl-flurazepam was measurable only during the early hours after a 30 mg dose and was not detectable after 24 hours. The major metabolite in blood was N1-desalkyl-flurazepam, which reached steady-state (plateau) levels after 7 to 10 days of dosing, at levels approximately 5- to 6-fold greater than the 24-hour levels observed on Day 1. The half-life of elimination of N1-desalkyl-flurazepam ranged from 47 to 100 hours. The major urinary metabolite is conjugated N1-hydroxyethyl-flurazepam which accounts for 22% to 55% of the dose. Less than 1% of the dose is excreted in the urine as N1-desalkyl-flurazepam.
This pharmacokinetic profile may be responsible for the clinical observation that flurazepam is increasingly effective on the second or third night of consecutive use and that for 1 or 2 nights after the drug is discontinued both sleep latency and total wake time may still be decreased.
The single dose pharmacokinetics of flurazepam were studied in 12 healthy geriatric subjects (aged 61 to 85 years). The mean elimination half-life of desalkyl-flurazepam was longer in elderly male subjects (160 hours) compared with younger male subjects (74 hours), while mean elimination half-life was similar in geriatric female subjects (120 hours) and younger female subjects (90 hours). After multiple dosing, mean steady-state plasma levels of desalkyl-flurazepam were higher in elderly male subjects (81 ng/mL) compared with younger male subjects (53 ng/mL), while values were similar between elderly female subjects (85 ng/mL) and younger female subjects (86 ng/mL). The mean washout half-life of desalkyl-flurazepam was longer in elderly male and female subjects (126 and 158 hours, respectively) compared with younger male and female subjects (111 and 113 hours, respectively).1
-
INDICATIONS AND USAGE
Flurazepam hydrochloride is a hypnotic agent useful for the treatment of insomnia characterized by difficulty in falling asleep, frequent nocturnal awakenings, and/or early morning awakening. Flurazepam hydrochloride capsules can be used effectively in patients with recurring insomnia or poor sleeping habits, and in acute or chronic medical situations requiring restful sleep. Sleep laboratory studies have objectively determined that flurazepam hydrochloride capsules are effective for at least 28 consecutive nights of drug administration. Since insomnia is often transient and intermittent, short-term use is usually sufficient. Prolonged use of hypnotics is usually not indicated and should only be undertaken concomitantly with appropriate evaluation of the patient.
-
CONTRAINDICATIONS
Flurazepam hydrochloride capsules are contraindicated in patients with known hypersensitivity to the drug.
Benzodiazepines may cause fetal damage when administered during pregnancy. An increased risk of congenital malformations associated with the use of diazepam and chlordiazepoxide during the first trimester of pregnancy has been suggested in several studies.
Flurazepam hydrochloride is contraindicated in pregnant women. Symptoms of neonatal depression have been reported; a neonate whose mother received 30 mg of flurazepam hydrochloride nightly for insomnia during the 10 days prior to delivery appeared hypotonic and inactive during the first 4 days of life. Serum levels of N1-desalkyl-flurazepam in the infant indicated transplacental circulation and implicate this long-acting metabolite in this case. If there is a likelihood of the patient becoming pregnant while receiving flurazepam, she should be warned of the potential risks to the fetus. Patients should be instructed to discontinue the drug prior to becoming pregnant. The possibility that a woman of childbearing potential may be pregnant at the time of institution of therapy should be considered.
-
WARNINGS
Because sleep disturbances may be the presenting manifestation of a physical and/or psychiatric disorder, symptomatic treatment of insomnia should be initiated only after a careful evaluation of the patient. The failure of insomnia to remit after 7 to 10 days of treatment may indicate the presence of a primary psychiatric and/or medical illness that should be evaluated. Worsening of insomnia or the emergence of new thinking or behavior abnormalities may be the consequence of an unrecognized psychiatric or physical disorder. Such findings have emerged during the course of treatment with sedative-hypnotic drugs. Because some of the important adverse effects of sedative-hypnotics appear to be dose related (see PRECAUTIONS and DOSAGE AND ADMINISTRATION), it is important to use the smallest possible effective dose, especially in the elderly.
Complex behaviors such as “sleep-driving” (i.e., driving while not fully awake after ingestion of a sedative-hypnotic, with amnesia for the event) have been reported. These events can occur in sedative-hypnotic-naïve as well as in sedative-hypnotic-experienced persons. Although behaviors such as sleep-driving may occur with sedative-hypnotics alone at therapeutic doses, the use of alcohol and other CNS depressants with sedative-hypnotics appears to increase the risk of such behaviors, as does the use of sedative-hypnotics at doses exceeding the maximum recommended dose. Due to the risk to the patients and the community, discontinuation of sedative-hypnotics should be strongly considered for patients who report a “sleep-driving” episode. Other complex behaviors (e.g., preparing and eating food, making phone calls or having sex) have been reported in patients who are not fully awake after taking a sedative-hypnotic. As with sleep-driving, patients usually do not remember these events.
Severe Anaphylactic and Anaphylactoid Reactions
Rare cases of angioedema involving the tongue, glottis or larynx have been reported in patients after taking the first or subsequent doses of sedative-hypnotics, including flurazepam. Some patients have had additional symptoms such as dyspnea, throat closing, or nausea and vomiting that suggest anaphylaxis. Some patients have required medical therapy in the emergency department. If angioedema involves the tongue, glottis or larynx, airway obstruction may occur and be fatal. Patients who develop angioedema after treatment with flurazepam should not be rechallenged with the drug.
Patients receiving flurazepam hydrochloride should be cautioned about possible combined effects with alcohol and other CNS depressants. Also, caution patients that an additive effect may occur if alcoholic beverages are consumed during the day following the use of flurazepam hydrochloride for nighttime sedation. The potential for this interaction continues for several days following discontinuance of flurazepam, until serum levels of psychoactive metabolites have declined.
Patients should also be cautioned about engaging in hazardous occupations requiring complete mental alertness such as operating machinery or driving a motor vehicle after ingesting the drug, including potential impairment of the performance of such activities which may occur the day following ingestion of flurazepam hydrochloride.
Clinical investigations of flurazepam hydrochloride have not been carried out in children. Therefore, the drug is not currently recommended for use in persons under 15 years of age.
Withdrawal symptoms of the barbiturate type have occurred after the discontinuation of benzodiazepines (see DRUG ABUSE AND DEPENDENCE).
-
PRECAUTIONS
Since the risk of the development of oversedation, dizziness, confusion and/or ataxia increases substantially with larger doses in elderly and debilitated patients, it is recommended that in such patients the dosage be limited to 15 mg. If flurazepam hydrochloride is to be combined with other drugs having known hypnotic properties or CNS-depressant effects, due consideration should be given to potential additive effects.
The usual precautions are indicated for severely depressed patients or those in whom there is any evidence of latent depression; particularly the recognition that suicidal tendencies may be present and protective measures may be necessary.
The usual precautions should be observed in patients with impaired renal or hepatic function and chronic pulmonary insufficiency.
Information for Patients“Sleep-Driving” and Other Complex Behavior
There have been reports of people getting out of bed after taking a sedative-hypnotic and driving their cars while not fully awake, often with no memory of the event. If a patient experiences such an episode, it should be reported to his or her doctor immediately, since “sleep-driving” can be dangerous. This behavior is more likely to occur when sedative-hypnotics are taken with alcohol or other central nervous system depressants (see WARNINGS). Other complex behaviors (e.g., preparing and eating food, making phone calls or having sex) have been reported in patients who are not fully awake after taking a sedative-hypnotic. As with sleep-driving, patients usually do not remember these events.
To assure the safe and effective use of benzodiazepines, patients should be informed that since benzodiazepines may produce psychological and physical dependence, it is advisable that they consult with their physician before either increasing the dose or abruptly discontinuing this drug.
Since the risk of the development of oversedation, dizziness, confusion and/or ataxia increases substantially with larger doses in elderly and debilitated patients, it is recommended that in such patients the dosage be limited to 15 mg. Staggering and falling have also been reported, particularly in geriatric patients.
Following single-dose administration of flurazepam, the elimination half-life for desalkyl-flurazepam was longer in elderly male subjects compared with younger male subjects, while values between elderly and young females were not significantly different. After multiple dosing, elimination half-life of desalkyl-flurazepam was longer in all elderly subjects compared with younger subjects, and mean steady-state serum concentrations were higher only in elderly male subjects relative to younger subjects (see CLINICAL PHARMACOLOGY: Geriatric Pharmacokinetics).
-
ADVERSE REACTIONS
Dizziness, drowsiness, light-headedness, staggering, ataxia and falling have occurred, particularly in elderly or debilitated persons. Severe sedation, lethargy, disorientation and coma, probably indicative of drug intolerance or overdosage, have been reported.
Also reported were headache, heartburn, upset stomach, nausea, vomiting, diarrhea, constipation, gastrointestinal pain, nervousness, talkativeness, apprehension, irritability, weakness, palpitations, chest pains, body and joint pains and genitourinary complaints. There have also been rare occurrences of leukopenia, granulocytopenia, sweating, flushes, difficulty in focusing, blurred vision, burning eyes, faintness, hypotension, shortness of breath, pruritus, skin rash, dry mouth, bitter taste, excessive salivation, anorexia, euphoria, depression, slurred speech, confusion, restlessness, hallucinations and elevated SGOT, SGPT, total and direct bilirubins, and alkaline phosphatase. Paradoxical reactions, e.g., excitement, stimulation and hyperactivity, have also been reported in rare instances.
-
DRUG ABUSE AND DEPENDENCE
Abuse and addiction are separate and distinct from physical dependence and tolerance. Abuse is characterized by misuse of the drug for non-medical purposes, often in combination with other psychoactive substances. Physical dependence is a state of adaptation that is manifested by a specific withdrawal syndrome that can be produced by abrupt cessation, rapid dose reduction, decreasing blood level of the drug and/or administration of an antagonist. Tolerance is a state of adaptation in which exposure to a drug induces changes that result in a diminution of one or more of the drug’s effects over time. Tolerance may occur to both the desired and undesired effects of the drug and may develop at different rates for different effects.
Addiction is a primary, chronic, neurobiological disease with genetic, psychosocial, and environmental factors influencing its development and manifestations. It is characterized by behaviors that include one or more of the following: impaired control over drug use, compulsive use, continued use despite harm, and craving. Drug addiction is a treatable disease, utilizing a multidisciplinary approach, but relapse is common.
Withdrawal symptoms, similar in character to those noted with barbiturates and alcohol (convulsions, tremor, abdominal and muscle cramps, vomiting and sweating), have occurred following abrupt discontinuance of benzodiazepines. The more severe withdrawal symptoms have usually been limited to those patients who had received excessive doses over an extended period of time. Generally milder withdrawal symptoms (e.g., dysphoria and insomnia) have been reported following abrupt discontinuance of benzodiazepines taken continuously at therapeutic levels for several months. Consequently, after extended therapy, abrupt discontinuation should generally be avoided and a gradual dosage tapering schedule followed. Addiction-prone individuals (such as drug addicts or alcoholics) should be under careful surveillance when receiving flurazepam hydrochloride or other psychotropic agents because of the predisposition of such patients to habituation and dependence.
-
OVERDOSAGE
Manifestations of flurazepam hydrochloride overdosage include somnolence, confusion and coma. Respiration, pulse and blood pressure should be monitored as in all cases of drug overdosage. General supportive measures should be employed, along with immediate gastric lavage. Intravenous fluids should be administered and an adequate airway maintained. Hypotension and CNS depression may be combated by judicious use of appropriate therapeutic agents. The value of dialysis has not been determined. If excitation occurs in patients following flurazepam hydrochloride overdosage, barbiturates should not be used. As with the management of intentional overdosage with any drug, it should be borne in mind that multiple agents may have been ingested.
Flumazenil, a specific benzodiazepine-receptor antagonist, is indicated for the complete or partial reversal of the sedative effects of benzodiazepines and may be useful in situations when an overdose with a benzodiazepine is known or suspected. Prior to the administration of flumazenil, necessary measures should be instituted to secure airway, ventilation, and intravenous access. Flumazenil is intended as an adjunct to, not as a substitute for, proper management of benzodiazepine overdose. Patients treated with flumazenil should be monitored for resedation, respiratory depression, and other residual benzodiazepine effects for an appropriate period after treatment. The prescriber should be aware of a risk of seizure in association with flumazenil treatment, particularly in long-term benzodiazepine users and in cyclic antidepressant overdose. The complete flumazenil package insert including CONTRAINDICATIONS, WARNINGS, and PRECAUTIONS should be consulted prior to use.
-
DOSAGE AND ADMINISTRATION
Dosage should be individualized for maximal beneficial effects. The usual adult dosage is 30 mg before retiring. In some patients, 15 mg may suffice. In elderly and/or debilitated patients, 15 mg is usually sufficient for a therapeutic response and it is therefore recommended that therapy be initiated with this dosage.
-
HOW SUPPLIED
Flurazepam Hydrochloride Capsules, USP are available containing either 15 mg or 30 mg of flurazepam hydrochloride, USP.
The 15 mg capsule is a hard-shell gelatin capsule with a white opaque cap and a powder blue opaque body filled with off-white to yellow powder. The capsule is axially printed with MYLAN over 4415 in black ink on both the cap and body. They are available as follows:
Bottles of 20
NDC 54868-0092-2
Bottles of 30
NDC 54868-0092-1
The 30 mg capsule is a hard-shell gelatin capsule with a powder blue opaque cap and a powder blue opaque body filled with off-white to yellow powder. The capsule is axially printed with MYLAN over 4430 in black ink on both the cap and body. They are available as follows:
Bottles of 10
NDC 54868-0093-0
Bottles of 30
NDC 54868-0093-1
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
Protect from light.
Dispense in a tight, light-resistant container as defined in the USP using a child-resistant closure.
PHARMACIST: Dispense a Medication Guide with each prescription.
- REFERENCES
-
MEDICATION GUIDE
FLURAZEPAM HYDROCHLORIDE
CAPSULES, USP
Flurazepam Hydrochloride Capsules USP, 15 mg and 30 mg are a Sedative-Hypnotic Indicated for Insomnia
Read this Medication Guide carefully before you start taking your medicine and each time you get more, since there may be new information. It does not contain all the information about your medicine that you may need to know, so please ask your doctor, nurse or pharmacist if you have any questions.
IMPORTANT
YOUR DOCTOR HAS PRESCRIBED THIS DRUG FOR YOUR USE ONLY. DO NOT LET ANYONE ELSE USE IT. KEEP THIS MEDICINE OUT OF THE REACH OF CHILDREN AND PETS. If a child puts a flurazepam hydrochloride capsule in his or her mouth or swallows it, call your local Poison Control Center or go immediately to the nearest emergency room.
What is the most important information I should know about sedative-hypnotic drugs?
After taking a sedative-hypnotic drug, you may get up out of bed while not being fully awake and do an activity that you do not know you are doing. The next morning, you may not remember that you did anything during the night. You have a higher chance for doing these activities if you drink alcohol or take other medications that make you sleepy with a sedative-hypnotic drug.
Reported activities include:
- driving a car ("sleep-driving")
- making and eating food
- talking on the phone
- having sex
- sleep-walking
Important:
-
Take a sedative-hypnotic drug exactly as prescribed:
- Do not take more sedative-hypnotic drugs than prescribed.
- Take the sedative-hypnotic drug right before you get in bed, not sooner.
-
Do not take a sedative-hypnotic drug if you:
- drink alcohol
- take other medicines that can make you sleepy. Talk to your doctor about all of your medicines. Your doctor will tell you if you can take a sedative-hypnotic drug with your other medicines
- cannot get a full night sleep
- Call your doctor right away if you find out that you have done any of the above activities after taking a sedative-hypnotic drug.
What is flurazepam?
Flurazepam is a sedative-hypnotic agent used to treat insomnia (difficulty falling asleep and staying asleep).
Who should not take flurazepam?
Do not use flurazepam if you are:
- allergic to anything in flurazepam hydrochloride capsules. (Being allergic may include having a rash, itching, swelling or breathing difficulties.) See the end of this Medication Guide for a complete list of ingredients in flurazepam hydrochloride capsules. In rare cases patient have had additional symptoms such as shortness of breath, throat closing, or nausea and vomiting that suggest an allergic reaction. Some patients have required medical therapy in the emergency department as these rare complications could be fatal. Patients who experience these symptoms should seek medical attention and discontinue taking the sedative-hypnotic drug.
- pregnant or intending to become pregnant. If a woman becomes pregnant while taking flurazepam, she should discontinue use immediately.
- under 15 years of age. Flurazepam has not been studied in children.
How should I take flurazepam?
Flurazepam comes as a capsule to take by mouth. You should take flurazepam, or other sedative-hypnotic medications, exactly as directed by your doctor. It usually is taken right before you get in bed, not sooner. If you forget to take flurazepam at bedtime, you are unable to fall asleep, and you will still be able to stay in bed for a full night’s sleep, you may take flurazepam at that time. Do not take a double dose of flurazepam to make up for a missed dose.
The smallest possible effective dose is suggested for elderly patients due to the risk of the development of oversedation, dizziness, confusion and/or loss of coordination.
Sleep problems are often temporary, requiring treatment for a very short time. You should not use flurazepam, or any other sedative-hypnotic medications, for long periods of time without talking to your doctor about the risks and benefits of prolonged use.
In the case of a suspected overdose, you should contact your local poison control center immediately.
What should I avoid while taking flurazepam?
Do not drink alcohol or take other medications that depress the central nervous system.
While taking flurazepam, do not engage in any hazardous occupations requiring complete mental alertness such as operating machinery or driving a car.
What are the possible or reasonably likely side effects of flurazepam?
Dizziness, drowsiness, light-headedness, staggering, loss of coordination and falling have occurred, particularly in elderly or debilitated persons. Severe sedation, lethargy, disorientation and coma, probably indicative of drug intolerance or overdosage, have been reported. Also reported are headache, heartburn, upset stomach, nausea, vomiting, diarrhea, constipation, nervousness, talking more than usual, anxiety, irritability, weakness, pounding heartbeat, chest pain, body and joint pains and difficulty urinating.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of flurazepam hydrochloride capsules
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. If you have any concerns about taking this, or any sedative-hypnotic medication, please ask your doctor. For detailed information regarding flurazepam hydrochloride capsules please consult the physician’s package insert. Do not use for conditions for which this medication was not prescribed. Do not give this medication to others.
What are the ingredients of flurazepam hydrochloride capsules, USP?
Active Ingredient: flurazepam hydrochloride, USP
Inactive Ingredients: colloidal silicon dioxide, magnesium stearate, microcrystalline cellulose, powdered cellulose and sodium lauryl sulfate. The empty gelatin capsule contains FD&C Blue No. 1, FD&C Red No. 3, gelatin and titanium dioxide. The imprinting ink contains black iron oxide, D&C Yellow No. 10 Aluminum Lake, FD&C Blue No. 1 Aluminum Lake, FD&C Blue No. 2 Aluminum Lake, FD&C Red No. 40 Aluminum Lake, propylene glycol and shellac glaze.
How should I store flurazepam hydrochloride capsules?
- Store flurazepam hydrochloride capsules at room temperature, 20° to 25°C (68° to 77°F).
- KEEP THIS MEDICINE OUT OF THE REACH OF CHILDREN. If a child accidentally takes flurazepam hydrochloride capsules, call your local Poison Control Center or go immediately to the nearest emergency room.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505REVISED MAY 2010
FLZ:R16mmt/MG:FLZ:R1
Relabeling and Repackaging by:
Physicians Total Care, Inc.
Tulsa, OK 74146
-
PRINCIPAL DISPLAY PANEL
CIV
FLURAZEPAM
HYDROCHLORIDE
CAPSULES, USP
15 mgPHARMACIST: Dispense the accompanying
Medication Guide to each patient.(Rx only)
Each capsule contains:
Flurazepam
hydrochloride, USP. . . . 15 mgDispense in a tight, light-resistant
container as defined in the USP
using a child-resistant closure.Keep container tightly closed.
Keep this and all medication out
of the reach of children.Store at 20° to 25°C (68° to 77°F).
[See USP Controlled Room
Temperature.]Protect from light.
Usual Adult Dosage: See accom-
panying prescribing information.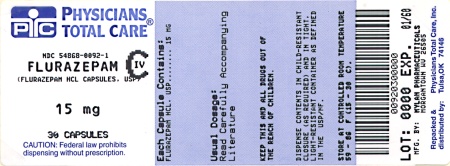
CIV
FLURAZEPAM
HYDROCHLORIDE
CAPSULES, USP
30 mgPHARMACIST: Dispense the accompanying
Medication Guide to each patient.(Rx only)
Each capsule contains:
Flurazepam
hydrochloride, USP. . . . 30 mgDispense in a tight, light-resistant
container as defined in the USP
using a child-resistant closure.Keep container tightly closed.
Keep this and all medication out
of the reach of children.Store at 20° to 25°C (68° to 77°F).
[See USP Controlled Room
Temperature.]Protect from light.
Usual Adult Dosage: See accom-
panying prescribing information.
-
INGREDIENTS AND APPEARANCE
FLURAZEPAM HYDROCHLORIDE
flurazepam hydrochloride capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:54868-0092(NDC:0378-4415) Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FLURAZEPAM HYDROCHLORIDE (UNII: 756RDM536M) (FLURAZEPAM - UNII:IHP475989U) FLURAZEPAM HYDROCHLORIDE 15 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POWDERED CELLULOSE (UNII: SMD1X3XO9M) SODIUM LAURYL SULFATE (UNII: 368GB5141J) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 3 (UNII: PN2ZH5LOQY) GELATIN (UNII: 2G86QN327L) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERROSOFERRIC OXIDE (UNII: XM0M87F357) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) FD&C RED NO. 40 (UNII: WZB9127XOA) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHELLAC (UNII: 46N107B71O) Product Characteristics Color white (white opaque) , blue (powder blue opaque) Score no score Shape CAPSULE Size 15mm Flavor Imprint Code MYLAN;4415 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54868-0092-1 30 in 1 BOTTLE, PLASTIC 2 NDC:54868-0092-2 20 in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA070345 05/08/1995 FLURAZEPAM HYDROCHLORIDE
flurazepam hydrochloride capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:54868-0093(NDC:0378-4430) Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FLURAZEPAM HYDROCHLORIDE (UNII: 756RDM536M) (FLURAZEPAM - UNII:IHP475989U) FLURAZEPAM HYDROCHLORIDE 30 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POWDERED CELLULOSE (UNII: SMD1X3XO9M) SODIUM LAURYL SULFATE (UNII: 368GB5141J) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 3 (UNII: PN2ZH5LOQY) GELATIN (UNII: 2G86QN327L) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERROSOFERRIC OXIDE (UNII: XM0M87F357) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) FD&C RED NO. 40 (UNII: WZB9127XOA) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHELLAC (UNII: 46N107B71O) Product Characteristics Color blue (powder blue opaque) Score no score Shape CAPSULE Size 15mm Flavor Imprint Code MYLAN;4430 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54868-0093-0 10 in 1 BOTTLE, PLASTIC 2 NDC:54868-0093-1 30 in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA070345 07/15/2002 06/30/2012 Labeler - Physicians Total Care, Inc. (194123980) Establishment Name Address ID/FEI Business Operations Physicians Total Care, Inc. 194123980 relabel(54868-0092, 54868-0093) , repack(54868-0092, 54868-0093)