Label: LACTULOSE solution
- NDC Code(s): 69067-010-15
- Packager: Foxland Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 9, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
LACTULOSE For Oral Solution is a synthetic disaccharide in the form of crystals for reconstitution prior to use for oral administration Each 10 g of lactulose contains less than 0.3 g galactose and lactose as a total sum. The pH range is 3.0 to 7.0.
Lactulose is a colonic acidifier which promotes laxation.
The chemical name for lactulose is 4-O-β-D-Galactopyranosyl-D-fructofuranose. It has the following structural formula:
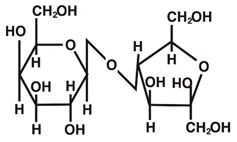
The molecular formula is C 12H 22O 11. The molecular weight is 342.30.
It is freely soluble in water. -
CLINICAL PHARMACOLOGY
LACTULOSE is poorly absorbed from the gastro-intestinal tract and no enzyme capable of hydrolysis of this disaccharide is present in human gastrointestinal tissue. As a result, oral doses of lactulose reach the colon virtually unchanged. In the colon, lactulose is broken down primarily to lactic acid, and also to small amounts of formic and acetic acids, by the action of colonic bacteria, which results in an increase in osmotic pressure and slight acidification of the colonic contents. This in turn causes an increase in stool water content and softens the stool.
Since lactulose does not exert its effect until it reaches the colon, and since transit time through the colon may be slow, 24 to 48 hours may be required to produce desired bowel movement.
Lactulose given orally to man and experimental animals resulted in only small amounts reaching the blood. Urinary excretion has been determined to be 3% or less and is essentially complete within 24 hours.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
A theoretical hazard may exist for patients being treated with lactulose who may be required to undergo electrocautery procedures during proctoscopy or colonoscopy. Accumulation of H 2 gas in significant concentration in the presence of an electrical spark may result in an explosive reaction. Although this complication has not been reported with lactulose, patients on lactulose therapy undergoing such procedures should have a thorough bowel cleansing with a non-fermentable solution. Insufflation of CO 2 as an additional safeguard may be pursued but is considered to be a redundant measure.
-
PRECAUTIONS
General
Since LACTULOSE For Oral Solution contains galactose and lactose (less than 0.3 g/10 g as a total sum), it should be used with caution in diabetics.
Information for Patients
In the event that an unusual diarrheal condition occurs, contact your physician.
Laboratory Tests
Elderly, debilitated patients who receive lactulose for more than six months should have serum electrolytes (potassium, chloride, carbon dioxide) measured periodically.
Drug Interactions
Results of preliminary studies in humans and rats suggest that nonabsorbable antacids given concurrently with lactulose may inhibit the desired lactulose-induced drop in colonic pH. Therefore, a possible lack of desired effect of treatment should be taken into consideration before such drugs are given concomitantly with lactulose.
Carcinogenesis, Mutagenesis, Impairment of Fertility
There are no known human data on long-term potential for carcinogenicity, mutagenicity, or impairment of fertility.
There are no known animal data on long-term potential for mutagenicity.
Administration of lactulose syrup in the diet of mice for 18 months in concentrations of 3 and 10 percent (v/w) did not produce any evidence of carcinogenicity.
In studies in mice, rats, and rabbits, doses of lactulose syrup up to 6 or 12 mL/kg/day produced no deleterious effects in breeding, conception, or parturition.
Pregnancy
Teratogenic Effects
Reproduction studies have been performed in mice, rats, and rabbits at doses up to 3 or 6 times the usual human oral dose and have revealed no evidence of impaired fertility or harm to the fetus due to lactulose. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
- ADVERSE REACTIONS
-
OVERDOSAGE
Signs and Symptoms
There have been no reports of accidental overdosage. In the event of overdosage, it is expected that diarrhea and abdominal cramps would be the major symptoms. Medication should be terminated.
- DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 10 Gram Packet Carton
-
INGREDIENTS AND APPEARANCE
LACTULOSE
lactulose solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:69067-010 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LACTULOSE (UNII: 9U7D5QH5AE) (LACTULOSE - UNII:9U7D5QH5AE) LACTULOSE 10 g in 10 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69067-010-15 15 in 1 CARTON 09/21/2018 1 10 g in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA074712 09/21/2018 Labeler - Foxland Pharmaceuticals, Inc. (079407828)


