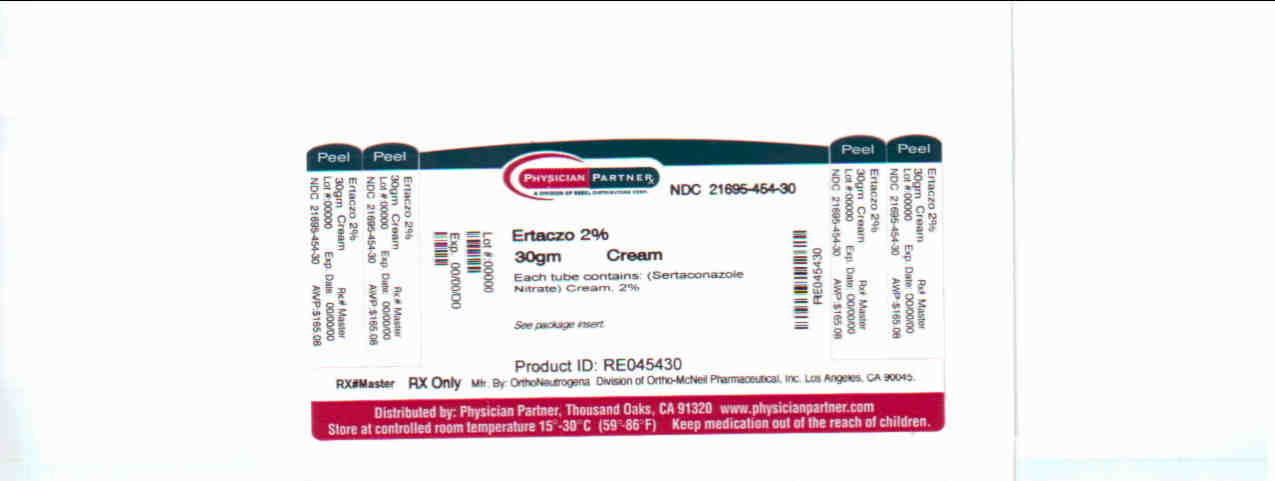
Label: ERTACZO cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 21695-454-30 - Packager: Rebel Distributors Corp.
- This is a repackaged label.
- Source NDC Code(s): 0062-1650
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 1, 2009
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
ERTACZO® (sertaconazole nitrate) Cream, 2%, contains the imidazole antifungal, sertaconazole nitrate. Sertaconazole nitrate contains one asymmetric carbon atom and exists as a racemic mixture of equal amounts of R and S enantiomers.
Sertaconazole nitrate is designated chemically as (±)-1-[2,4-dichloro-β-[(7-chlorobenzo-[b]thien-3-yl)methoxy]phenethyl]imidazole nitrate. It has a molecular weight of 500.8. The molecular formula is C20H15Cl3N2OS • HNO3, and the structural formula is as follows:
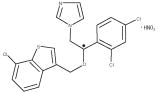
Sertaconazole nitrate is a white or almost white powder. It is practically insoluble in water, soluble in methanol, sparingly soluble in alcohol and in methylene chloride. Each gram of ERTACZO® Cream, 2%, contains 17.5 mg of sertaconazole (as sertaconazole nitrate, 20 mg) in a white cream base of ethylene glycol and polyethylene glycol palmitostearate, glyceryl isostearate, light mineral oil, methylparaben, polyoxyethylened saturated glycerides and glycolized saturated glycerides, sorbic acid and purified water.
-
CLINICAL PHARMACOLOGY
Pharmacokinetics: In a multiple dose pharmacokinetic study that included 5 male patients with interdigital tinea pedis (range of diseased area, 42 - 140 cm2; mean, 93 cm2), ERTACZO® Cream, 2%, was topically applied every 12 hours for a total of 13 doses to the diseased skin (0.5 grams sertaconazole nitrate per 100 cm2 ). Sertaconazole concentrations in plasma measured by serial blood sampling for 72 hours after the thirteenth dose were below the limit of quantitation (2.5 ng/mL) of the analytical method used.
Microbiology: Sertaconazole is an antifungal that belongs to the imidazole class of antifungals. While the exact mechanism of action of this class of antifungals is not known, it is believed that they act primarily by inhibiting the cytochrome P450-dependent synthesis of ergosterol. Ergosterol is a key component of the cell membrane of fungi, and lack of this component leads to fungal cell injury primarily by leakage of key constituents in the cytoplasm from the cell.
-
CLINICAL STUDIES
In two randomized, double-blind, clinical trials, patients 12 years and older with interdigital tinea pedis applied either ERTACZO® Cream, 2%, or vehicle, twice daily for four weeks. Patients with moccasin-type (plantar) tinea pedis and/or onychomycosis were excluded from the study. Two weeks after completion of therapy (six weeks after beginning therapy), patients were evaluated for signs and symptoms related to interdigital tinea pedis.
Treatment outcomes are summarized in the table below.
Treatment Outcomes as Percent (%) of Total Subjects * Complete Cure - Patients who had complete clearing of signs and symptoms and Mycological Cure.
** Effective Treatment - Patients who had minimal residual signs and symptoms of interdigital tinea pedis and Mycological Cure.
*** Mycological Cure - Patients who had both negative microscopic KOH preparation and a negative fungal culture.
Study 1 Study 2 Sertaconazole Vehicle Sertaconazole Vehicle Complete Cure*
(Primary Efficacy Variable)13/99 (13.1%) 3/92 (3.3%) 28/103 (27.2%) 5/103 (4.9%) Effective Treatment** 32/99 (32.3%) 11/92 (12.0%) 52/103 (50.5%) 16/103 (15.5%) Mycological Cure*** 49/99 (49.5%) 18/92 (19.6%) 71/103 (68.9%) 20/103 (19.4%) In clinical trials, complete cure in sertaconazole treated patients was achieved in 32 of 160 (20%) patients with Trichophyton rubrum, in 7 of 28 (25%) patients with Trichophyton mentagrophytes and in 2 of 13 (15%) patients with Epidermophyton floccosum.
-
INDICATIONS AND USAGE
ERTACZO® (sertaconazole nitrate) Cream, 2%, is indicated for the topical treatment of interdigital tinea pedis in immunocompetent patients 12 years of age and older, caused by: Trichophyton rubrum, Trichophyton mentagrophytes, and Epidermophyton floccosum (see CLINICAL STUDIES Section).
- CONTRAINDICATIONS
- WARNINGS
-
PRECAUTIONS
General: ERTACZO® Cream, 2%, is for use on the skin only. If irritation or sensitivity develops with the use of ERTACZO® Cream, 2%, treatment should be discontinued and appropriate therapy instituted.
Diagnosis of the disease should be confirmed either by direct microscopic examination of infected superficial epidermal tissue in a solution of potassium hydroxide or by culture on an appropriate medium.
Physicians should exercise caution when prescribing ERTACZO® Cream, 2%, to patients known to be sensitive to imidazole antifungals, since cross-reactivity may occur.
Information for Patients: The patient should be instructed to:
- Use ERTACZO® Cream, 2%, as directed by the physician. The hands should be washed after applying the medication to the affected area(s). Avoid contact with the eyes, nose, mouth and other mucous membranes. ERTACZO® Cream, 2%, is for external use only.
- Dry the affected area(s) thoroughly before application, if you wish to use ERTACZO® Cream, 2%, after bathing.
- Use the medication for the full treatment time recommended by the physician, even though symptoms may have improved. Notify the physician if there is no improvement after the end of the prescribed treatment period, or sooner, if the condition worsens.
- Inform the physician if the area of application shows signs of increased irritation, redness, itching, burning, blistering, swelling or oozing.
- Avoid the use of occlusive dressings unless otherwise directed by the physician.
- Do not use this medication for any disorder other than that for which it was prescribed.
Drug/Laboratory Test Interactions: Potential interactions between ERTACZO® Cream, 2%, and other drugs or laboratory tests have not been systematically evaluated.
Carcinogenesis, Mutagenesis, Impairment of Fertility: Long-term studies to evaluate the carcinogenic potential of sertaconazole nitrate have not been conducted. No clastogenic potential was observed in a mouse micronucleus test. Sertaconazole nitrate was considered negative for sister chromatid exchange (SCE) in the in vivo mouse bone marrow SCE assay. There was no evidence that sertaconazole nitrate induced unscheduled DNA synthesis in rat primary hepatocyte cultures. Sertaconazole nitrate exhibited no toxicity or adverse effects on reproductive performance or fertility of male or female rats given up to 60 mg/kg/day orally by gastric intubation (16 times the maximum recommended human dose based on a body surface area comparison).
Pregnancy: Teratogenic Effects. Pregnancy Category C: Oral reproduction studies in rats and rabbits did not produce any evidence of maternal toxicity, embryotoxicity or teratogenicity of sertaconazole nitrate at an oral dose of 160 mg/kg/day (40 times (rats) and 80 times (rabbits) the maximum recommended human dose on a body surface area comparison). In an oral peri-postnatal study in rats, a reduction in live birth indices and an increase in the number of still-born pups was seen at 80 and 160 mg/kg/day.
There are no adequate and well-controlled studies that have been conducted on topically applied ERTACZO® Cream, 2%, in pregnant women. Because animal reproduction studies are not always predictive of human response, ERTACZO® Cream, 2%, should be used during pregnancy only if clearly needed.
Nursing Mothers: It is not known if sertaconazole is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when prescribing ERTACZO® Cream, 2%, to a nursing woman.
-
ADVERSE EVENTS
In clinical trials, cutaneous adverse events occurred in 7 of 297 (2%) patients (2 of them severe) receiving ERTACZO® Cream, 2%, and in 7 of 291 (2%) patients (2 of them severe) receiving vehicle. These reported cutaneous adverse events included contact dermatitis, dry skin, burning skin, application site reaction and skin tenderness.
In a dermal sensitization study, 8 of 202 evaluable patients tested with ERTACZO® Cream, 2%, and 4 of 202 evaluable patients tested with vehicle, exhibited a slight erythematous reaction in the challenge phase. There was no evidence of cumulative irritation or contact sensitization in a repeated insult patch test involving 202 healthy volunteers. In non-US post-marketing surveillance for ERTACZO® Cream, 2%, the following cutaneous adverse events were reported: contact dermatitis, erythema, pruritus, vesiculation, desquamation, and hyperpigmentation.
- OVERDOSAGE
-
DOSAGE AND ADMINISTRATION
In the treatment of interdigital tinea pedis, ERTACZO® Cream, 2%, should be applied twice daily for 4 weeks. Sufficient ERTACZO® Cream, 2%, should be applied to cover both the affected areas between the toes and the immediately surrounding healthy skin of patients with interdigital tinea pedis. If a patient shows no clinical improvement 2 weeks after the treatment period, the diagnosis should be reviewed.
-
HOW SUPPLIED
ERTACZO® Cream, 2%, is supplied in tubes in the following size:
- 30-gram tube NDC 21695-454-30
Store at 25°C (77°F); excursions permitted to 15º-30°C (59º-86°F) [see USP Controlled Room Temperature].
Rx only.
Patent No. 5,135,943
Distributed By: OrthoNeutrogena
DIVISION OF ORTHO-McNEIL PHARMACEUTICAL, INC.
Los Angeles, CA 90045Repackaged By: Rebel Distributors
Thousand Oaks, CA 91320Rev November 2005
128354 - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ERTACZO
ertaczo creamProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:21695-454(NDC:0062-1650) Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Sertaconazole Nitrate (UNII: 1DV05410M5) (Sertaconazole - UNII:72W71I16EG) Sertaconazole Nitrate 20 mg in 1 g Inactive Ingredients Ingredient Name Strength ETHYLENE GLYCOL (UNII: FC72KVT52F) POLYETHYLENE GLYCOL (UNII: 3WJQ0SDW1A) Light mineral oil (UNII: N6K5787QVP) Methylparaben (UNII: A2I8C7HI9T) Sorbic acid (UNII: X045WJ989B) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:21695-454-30 30 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA021385 12/10/2003 Labeler - Rebel Distributors Corp. (118802834) Establishment Name Address ID/FEI Business Operations Rebel Distributors Corp. 118802834 repack, relabel