HEB NASAL- phenylephrine hydrochloride spray
H E B
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
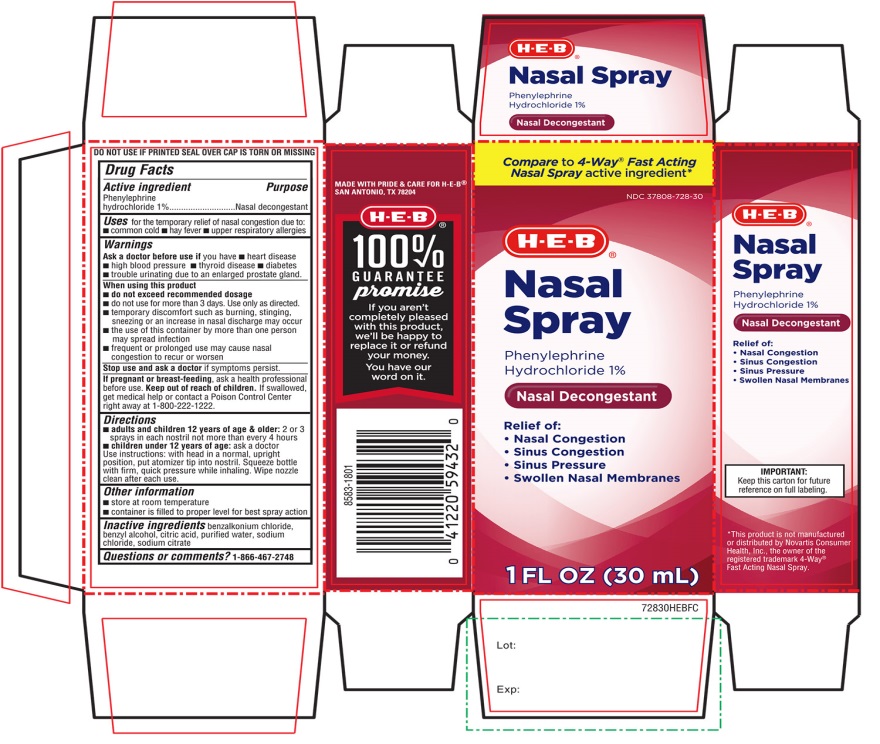
H.E.B. Nasal Spray Drug Facts
Uses
For the temporary relief of nasal congestion due to:
- •
- common cold
- •
- hay fever
- •
- upper respiratory allergies
Warnings
Ask a doctor before use if you have
- •
- heart disease
- •
- high blood pressure
- •
- thyroid disease
- •
- diabetes
- •
- trouble urinating due to an enlarged prostate gland
When using this product
- •
- do not exceed recommended dosage
- •
- do not use for more than 3 days. use only as directed.
- •
- temporary discomfort such as burning, stinging, sneezing or an increase in nasal discharge may occur
- •
- the use of this container by more than one person may spread infection
- •
- frequent or prolonged use may cause nasal congestion to recur or worsen
Directions
- •
- adults and children 12 years of age & older: 2 or 3 sprays in each nostril not more often than every 4 hours
- •
- children under 12 years of age: ask a doctor
- •
- Use instructions: with head in a normal, upright position, put atomizer tip into nostril. Squeeze bottle with firm, quick pressure while inhaling. Wipe nozzle clean after each use.
Other information
- •
- store at room Temperature
- •
- container is filled to proper level for best spray action
Inactive ingredients
benzalkonium chloride, benzyl alcohol, citric acid, purified water, sodium chloride, sodium citrate
Principal Display Panel
Compare to 4-Way® Fast Acting Nasal Spray active ingredient*
NDC 37808-728-30
H.E.B®
Nasal Spray
Phenylephrine Hydrochloride 1 %
Nasal Decongestant
Relief of:
- •
- Nasal Congestion
- •
- Sinus Congestion
- •
- Sinus Pressure
- •
- Swollen Nasal Membranes
1 FL OZ (30mL)
DO NOT USE IF PRINTED SEAL OVER CAP IS TORN OR MISSING
MADE WITH PRIDE & CARE FOR H-E-B®
SAN ANTONIO, TX 78204
H-E-B®
100% GUARANTEE promise
If you aren’t completely pleases with this product, we’ll be happy to replace it or refund your money.
You have our word on it.
Keep this carton for future reference on full labeling.
*This product is not manufactured or distributed by Novartis Consumer Health, Inc.,
the owner of the registered trademark 4-Way®
Fast Acting Nasal Spray.
| HEB NASAL
phenylephrine hydrochloride spray |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - H E B (007924756) |