Label: LISINOPRIL AND HYDROCHLOROTHIAZIDE tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 66336-572-90 - Packager: Dispensing Solutions, Inc.
- This is a repackaged label.
- Source NDC Code(s): 68180-518
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 7, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
BOXED WARNING
(What is this?)
USE IN PREGNANCY
When used in pregnancy during the second and third trimesters, ACE inhibitors can cause injury and even death to the developing fetus. When pregnancy is detected, lisinopril and hydrochlorothiazide tablets should be discontinued as soon as possible. See WARNINGS, Pregnancy, Lisinopril, Fetal/Neonatal Morbidity and Mortality. -
DESCRIPTION
Lisinopril and hydrochlorothiazide tablets combines an angiotensin converting enzyme inhibitor, lisinopril, and a diuretic, hydrochlorothiazide.
Lisinopril, a synthetic peptide derivative, is an oral long-acting angiotensin converting enzyme inhibitor. It is chemically described as (S)-1-[N2-(1-carboxy-3-phenylpropyl)-L-lysyl]-L-proline dihydrate. Its empirical formula is C21H31N3O5•2H2O and its structural formula is: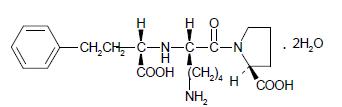
Lisinopril is a white to off-white, crystalline powder, with a molecular weight of 441.52. It is soluble in water, sparingly soluble in methanol, and practically insoluble in ethanol.
Hydrochlorothiazide is 6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide. Its empirical formula is C7H8ClN3O4S2 and its structural formula is: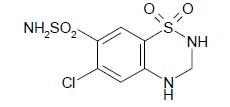
Hydrochlorothiazide is a white, or practically white, crystalline powder with a molecular weight of 297.73, which is slightly soluble in water, but freely soluble in sodium hydroxide solution.
Lisinopril and hydrochlorothiazide tablets are available for oral use in three tablet combinations of lisinopril with hydrochlorothiazide: lisinopril and hydrochlorothiazide tablets 10 mg/12.5 mg, containing 10 mg lisinopril and 12.5 mg hydrochlorothiazide, lisinopril and hydrochlorothiazide tablets 20 mg/12.5 mg, containing 20 mg lisinopril and 12.5 mg hydrochlorothiazide and lisinopril and hydrochlorothiazide tablets 20 mg/25 mg, containing 20 mg lisinopril and 25 mg hydrochlorothiazide.
Inactive ingredients are dibasic calcium phosphate, magnesium stearate, mannitol, pregelatinized starch and starch. Lisinopril and hydrochlorothiazide tablets 10 mg/12.5 mg also contains FD and C Blue No. 2 Aluminum Lake. Lisinopril and hydrochlorothiazide tablets 20 mg/12.5 mg also contains yellow iron oxide and lisinopril and hydrochlorothiazide tablets 20 mg/25 mg also contain red iron oxide. -
INDICATIONS AND USAGE
Lisinopril and hydrochlorothiazide tablets are indicated for the treatment of hypertension.
These fixed-dose combinations are not indicated for initial therapy (see DOSAGE AND ADMINISTRATION).
In using lisinopril and hydrochlorothiazide tablets, consideration should be given to the fact that an angiotensin converting enzyme inhibitor, captopril, has caused agranulocytosis, particularly in patients with renal impairment or collagen vascular disease, and that available data are insufficient to show that lisinopril does not have a similar risk. (See WARNINGS.)
In considering use of lisinopril and hydrochlorothiazide tablets, it should be noted that Black patients receiving ACE inhibitors have been reported to have a higher incidence of angioedema compared to non-Blacks. (See WARNINGS, Head and Neck Angioedema.) -
CONTRAINDICATIONS
Lisinopril and hydrochlorothiazide is contraindicated in patients who are hypersensitive to any component of this product and in patients with a history of angioedema related to previous treatment with an angiotensin converting enzyme inhibitor and in patients with hereditary or idiopathic angioedema. Because of the hydrochlorothiazide component, this product is contraindicated in patients with anuria or hypersensitivity to other sulfonamide-derived drugs.
-
ADVERSE REACTIONS
Lisinopril and hydrochlorothiazide has been evaluated for safety in 930 patients, including 100 patients treated for 50 weeks or more.
In clinical trials with lisinopril and hydrochlorothiazide no adverse experiences peculiar to this combination drug have been observed. Adverse experiences that have occurred have been limited to those that have been previously reported with lisinopril or hydrochlorothiazide.
The most frequent clinical adverse experiences in controlled trials (including open label extensions) with any combination of lisinopril and hydrochlorothiazide were: dizziness (7.5 percent), headache (5.2 percent), cough (3.9 percent), fatigue (3.7 percent) and orthostatic effects (3.2 percent), all of which were more common than in placebo-treated patients. Generally, adverse experiences were mild and transient in nature; but see WARNINGSregarding angioedema and excessive hypotension or syncope. Discontinuation of therapy due to adverse effects was required in 4.4 percent of patients, principally because of dizziness, cough, fatigue and muscle cramps.
Adverse experiences occurring in greater than one percent of patients treated with lisinopril plus hydrochlorothiazide in controlled clinical trials are shown below.Percent of Patients in Controlled Studies
Clinical adverse experiences occurring in 0.3 to 1.0 percent of patients in controlled trials included: Body as a Whole: Chest pain, abdominal pain, syncope, chest discomfort, fever, trauma, virus infection. Cardiovascular: Palpitation, orthostatic hypotension. Digestive: Gastrointestinal cramps, dry mouth, constipation, heartburn. Musculoskeletal: Back pain, shoulder pain, knee pain, back strain, myalgia, foot pain. Nervous/Psychiatric: Decreased libido, vertigo, depression, somnolence. Respiratory: Common cold, nasal congestion, influenza, bronchitis, pharyngeal pain, dyspnea, pulmonary congestion, chronic sinusitis, allergic rhinitis, pharyngeal discomfort. Skin: Flushing, pruritus, skin inflammation, diaphoresis. Special Senses: Blurred vision, tinnitus, otalgia. Urogenital: Urinary tract infection.
Lisinopril-Hydrochlorothiazide
(n=930)
Incidence
(discontinuation)
Placebo
(n=207)
IncidenceDizziness
7.5 (.8)
1.9
Headache
5.2 (0.3)
19
Cough
3.9 (0.6)
1.0
Fatigue
3.7 (0.4)
1.0
Orthostatic Effects
3.2 (0.1)
1.0
Diarrhea
2.5 (0.2)
2.4
Nausea
2.2 (0.1)
2.4
Upper Respiratory Infection
2.2 (0.0)
0.0
Muscle Cramps
2.0 (0.4)
0.5
Asthenia
1.8 (0.2)
1.0
Parasthesia
1.5 (0.1)
0.0
Hypotension
1.4 (0.3)
0.5
Vomiting
1.4 (0.1)
0.5
Dyspepsia
1.3 (0.0)
0.0
Rash
1.2 (0.1)
0.5
Impotence
1.2 (0.3)
0.0
Angioedema: Angioedema has been reported in patients receiving lisinopril and hydrochlorothiazide, with an incidence higher in Black than in non-Black patients. Angioedema associated with laryngeal edema may be fatal. If angioedema of the face, extremities, lips, tongue, glottis and/or larynx occurs, treatment with lisinopril and hydrochlorothiazide should be discontinued and appropriate therapy instituted immediately. In rare cases, intestinal angioedema has been reported with angiotensin converting enzyme inhibitors including lisinopril. (See WARNINGS.)
Hypotension: In clinical trials, adverse effects relating to hypotension occurred as follows: hypotension (1.4), orthostatic hypotension (0.5), other orthostatic effects (3.2). In addition syncope occurred in 0.8 percent of patients. (See WARNINGS.)
Cough: See PRECAUTIONS, Cough.
Clinical Laboratory Test Findings
Serum Electrolytes: See PRECAUTIONS.
Creatinine, Blood Urea Nitrogen: Minor reversible increases in blood urea nitrogen and serum creatinine were observed in patients with essential hypertension treated with lisinopril and hydrochlorothiazide. More marked increases have also been reported and were more likely to occur in patients with renal artery stenosis. (See PRECAUTIONS.)
Serum Uric Acid, Glucose, Magnesium, Cholesterol, Triglycerides and Calcium: See PRECAUTIONS. -
OVERDOSAGE
No specific information is available on the treatment of overdosage with lisinopril and hydrochlorothiazide. Treatment is symptomatic and supportive. Therapy with lisinopril and hydrochlorothiazide should be discontinued and the patient observed closely. Suggested measures include induction of emesis and/or gastric lavage, and correction of dehydration, electrolyte imbalance and hypotension by established procedures.
Lisinopril
Following a single oral dose of 20 mg/kg, no lethality occurred in rats and death occurred in one of 20 mice receiving the same dose. The most likely manifestation of overdosage would be hypotension, for which the usual treatment would be intravenous infusion of normal saline solution.
Lisinopril can be removed by hemodialysis. (See WARNINGS, Anaphylactoid reactions during membrane exposure.)Hydrochlorothiazide
Oral administration of a single oral dose of 10 mg/kg to mice and rats was not lethal. The most common signs and symptoms observed are those caused by electrolyte depletion (hypokalemia, hypochloremia, hyponatremia) and dehydration resulting from excessive diuresis. If digitalis has also been administered, hypokalemia may accentuate cardiac arrhythmias.
-
DOSAGE AND ADMINISTRATION
Lisinopril is an effective treatment of hypertension in once-daily doses of 10 to 80 mg, while hydrochlorothiazide is effective in doses of 12.5 to 50 mg. In clinical trials of lisinopril/hydrochlorothiazide combination therapy using lisinopril doses of 10 to 80 mg and hydrochlorothiazide doses of 6.25 to 50 mg, the antihypertensive response rates generally increased with increasing dose of either component.
The side effects (see WARNINGS) of lisinopril are generally rare and apparently independent of dose; those of hydrochlorothiazide are a mixture of dose-dependent phenomena (primarily hypokalemia) and dose-independent phenomena (e.g., pancreatitis), the former much more common than the latter. Therapy with any combination of lisinopril and hydrochlorothiazide will be associated with both sets of dose-independent side effects, but addition of lisinopril in clinical trials blunted the hypokalemia normally seen with diuretics.
To minimize dose-independent side effects, it is usually appropriate to begin combination therapy only after a patient has failed to achieve the desired effect with monotherapy.Dose Titration Guided by Clinical Effect
A patient whose blood pressure is not adequately controlled with either lisinopril or hydrochlorothiazide monotherapy may be switched to lisinopril and hydrochlorothiazide tablets 10 mg/12.5 mg or lisinopril and hydrochlorothiazide tablets 20 mg/12.5 mg. Further increases of either or both components could depend on clinical response. The hydrochlorothiazide dose should generally not be increased until 2-3 weeks have elapsed. Patients whose blood pressures are adequately controlled with 25 mg of daily hydrochlorothiazide, but who experience significant potassium loss with this regimen, may achieve similar or greater blood pressure control with less potassium loss if they are switched to lisinopril and hydrochlorothiazide tablets 10 mg/12.5 mg. Dosage higher than lisinopril 80 mg and hydrochlorothiazide 50 mg should not be used.
Use in Renal Impairment
The usual regimens of therapy with lisinopril and hydrochlorothiazide tablets need not be adjusted as long as the patient's creatinine clearance is >30 mL/min/1.73 m2 (serum creatinine approximately (3 mg/dL or 265 µmol/L). In patients with more severe renal impairment, loop diuretics are preferred to thiazides, so lisinopril and hydrochlorothiazide tablets are not recommended (see WARNINGS, Anaphylactoid reactions during membrane exposure).
-
HOW SUPPLIED
Lisinopril and Hydrochlorothiazide Tablets USP, 10 mg/12.5 mg are blue, hexagonal tablets, with "LL" debossed on one side and "B01" on other side.
They are supplied as follows:
NDC 68180-518-01 – 100’s bottle
NDC 68180-518-02 – 500’s bottle
Lisinopril and Hydrochlorothiazide Tablets USP, 20 mg/12.5 mg are yellow, round tablets, with "LL" debossed on one side and "B02" on other side.
They are supplied as follows:
NDC 68180-519-01 – 100’s bottle
NDC 68180-519-02 – 500’s bottle
Lisinopril and Hydrochlorothiazide Tablets USP, 20 mg/25 mg are peach, round tablets, with "LL" debossed on one side and "B03" on other side.
They are supplied as follows:
NDC 68180-520-01 – 100’s bottle
NDC 68180-520-02 – 500’s bottle
Storage
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature]. Protect from excessive light and humidity. - SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LISINOPRIL AND HYDROCHLOROTHIAZIDE
lisinopril and hydrochlorothiazide tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:66336-572(NDC:68180-518) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROCHLOROTHIAZIDE (UNII: 0J48LPH2TH) (HYDROCHLOROTHIAZIDE - UNII:0J48LPH2TH) HYDROCHLOROTHIAZIDE 12.5 mg LISINOPRIL (UNII: E7199S1YWR) (LISINOPRIL - UNII:E7199S1YWR) LISINOPRIL 10 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) MANNITOL (UNII: 3OWL53L36A) STARCH, CORN (UNII: O8232NY3SJ) MAGNESIUM STEARATE (UNII: 70097M6I30) STARCH, PREGELATINIZED CORN (UNII: O8232NY3SJ) Product Characteristics Color blue Score no score Shape HEXAGON (6 sided) Size 7mm Flavor Imprint Code LL;B01 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66336-572-90 90 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA077912 10/02/2006 Labeler - Dispensing Solutions, Inc. (066070785) Establishment Name Address ID/FEI Business Operations Dispensing Solutions, Inc. 066070785 relabel, repack


