Label: CLOBETASOL PROPIONATE spray
- NDC Code(s): 70771-1080-6, 70771-1080-7
- Packager: Zydus Lifesciences Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 31, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
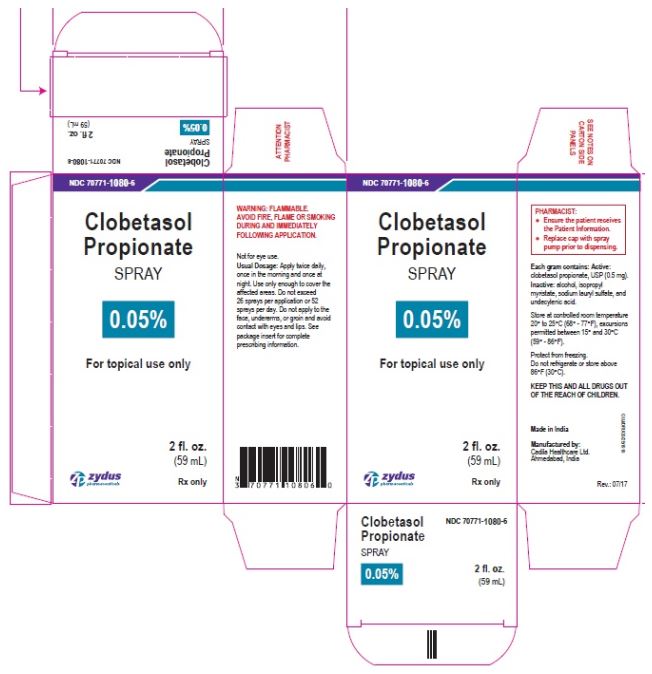
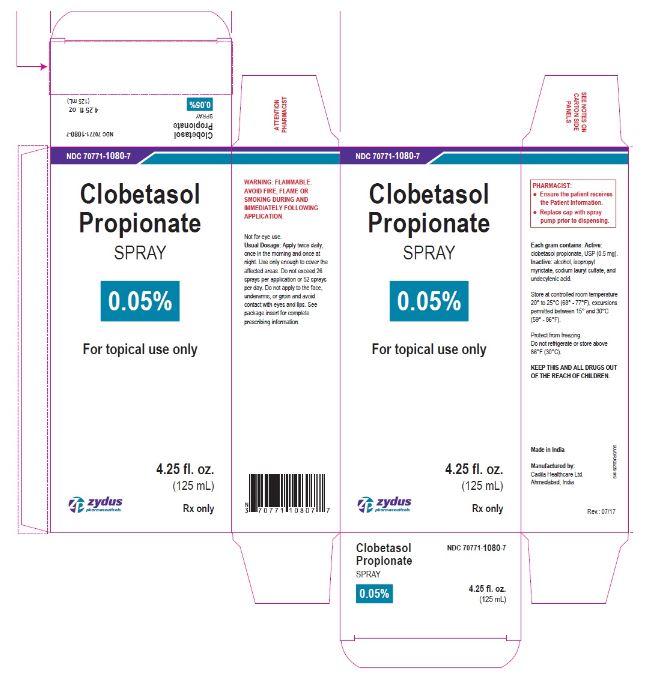
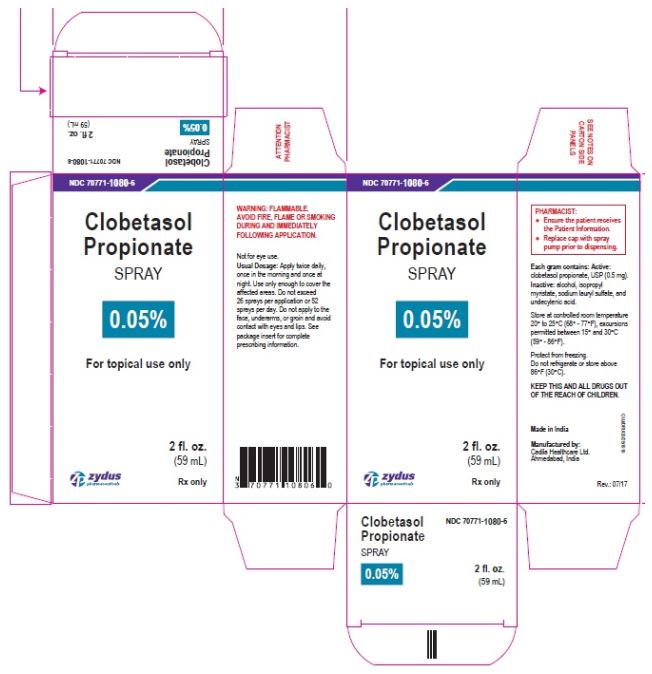
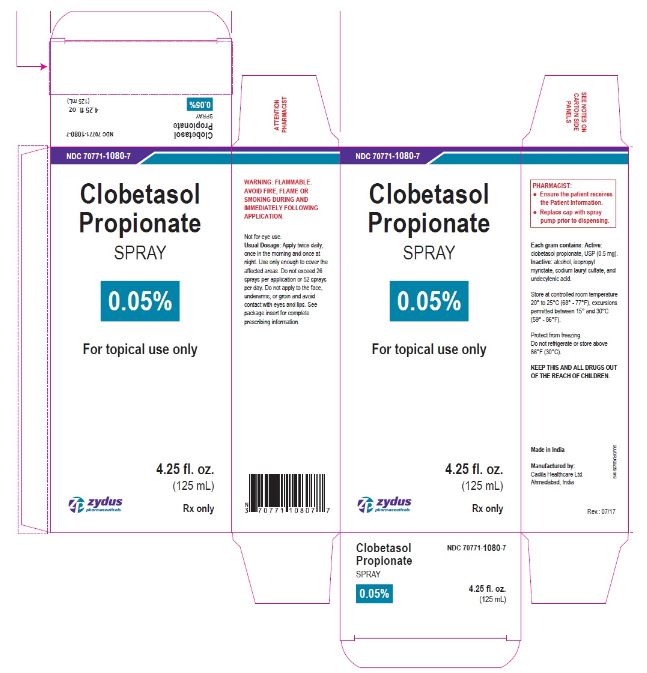
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CLOBETASOL PROPIONATE
clobetasol propionate sprayProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70771-1080 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CLOBETASOL PROPIONATE (UNII: 779619577M) (CLOBETASOL - UNII:ADN79D536H) CLOBETASOL PROPIONATE 0.05 g in 1 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) SODIUM LAURYL SULFATE (UNII: 368GB5141J) UNDECYLENIC ACID (UNII: K3D86KJ24N) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70771-1080-7 1 in 1 CARTON 07/12/2017 1 125 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:70771-1080-6 1 in 1 CARTON 07/12/2017 2 59 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA206378 07/12/2017 Labeler - Zydus Lifesciences Limited (918596198) Registrant - Zydus Lifesciences Limited (918596198) Establishment Name Address ID/FEI Business Operations Zydus Lifesciences Limited 650650802 ANALYSIS(70771-1080) , MANUFACTURE(70771-1080)