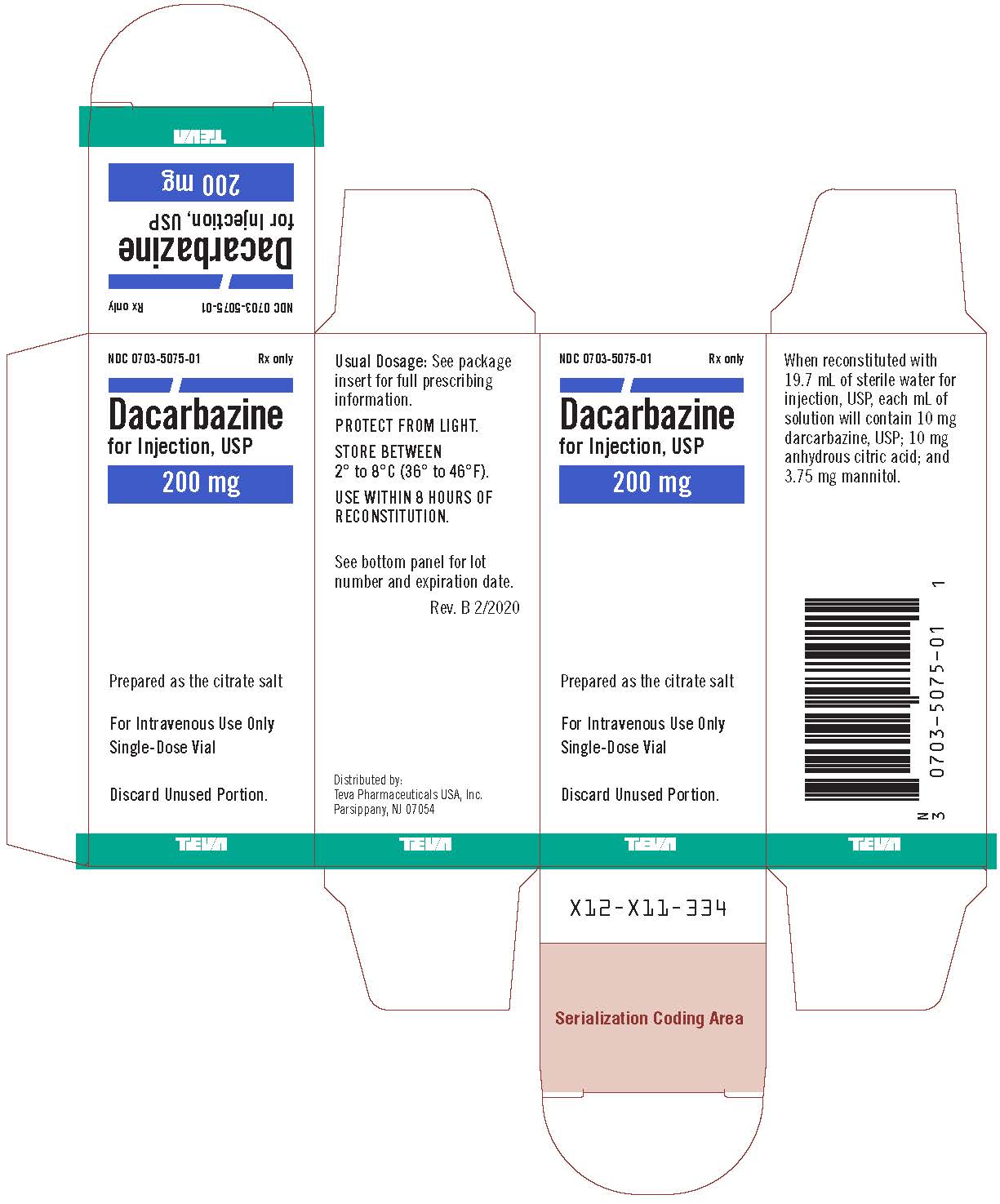
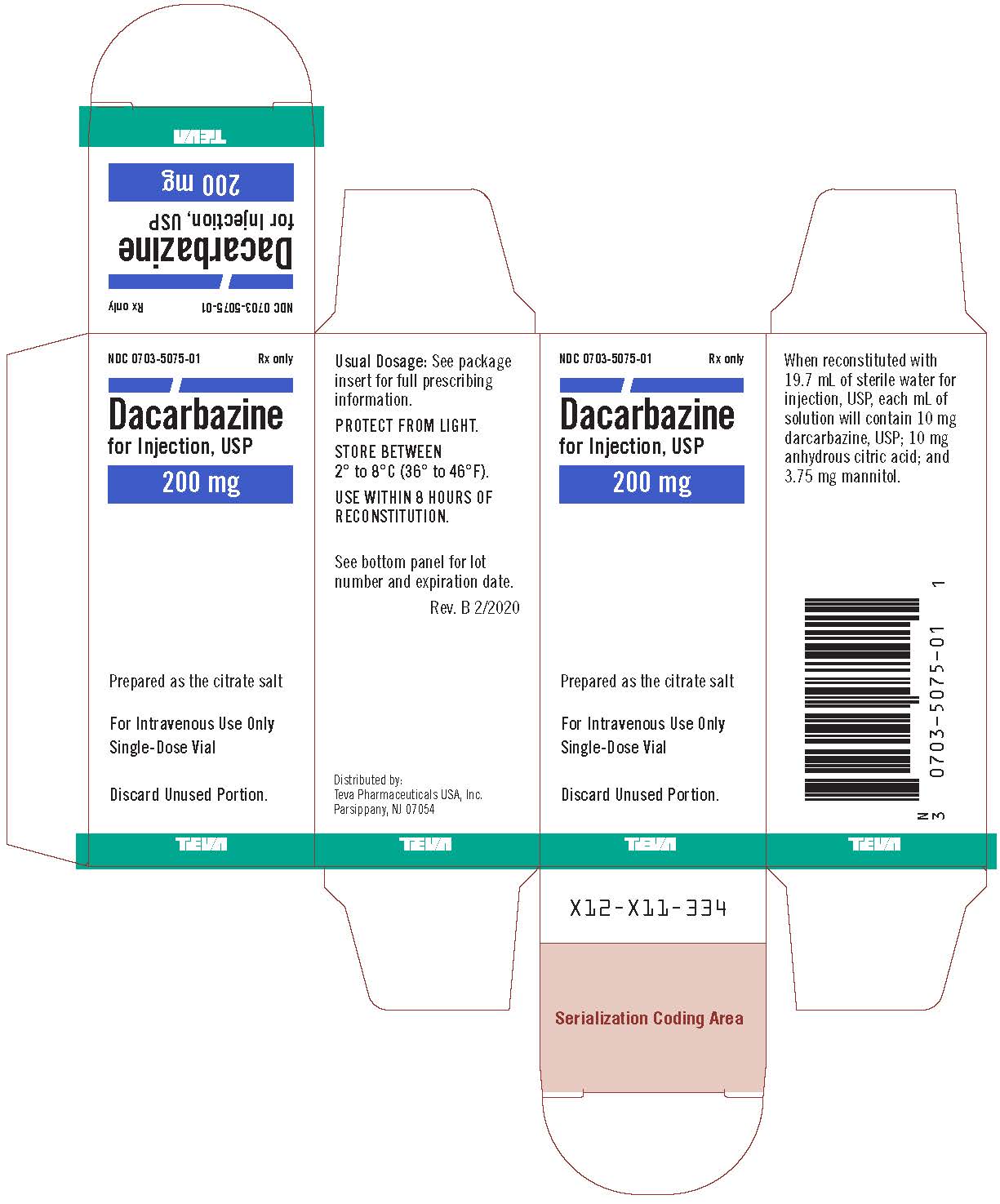
Label: DACARBAZINE injection, powder, for solution
- NDC Code(s): 0703-5075-01, 0703-5075-03
- Packager: Teva Parenteral Medicines, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 28, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
BOXED WARNING
(What is this?)
WARNING
It is recommended that dacarbazine for injection be administered under the supervision of a qualified physician experienced in the use of cancer chemotherapeutic agents.
- Hemopoietic depression is the most common toxicity with dacarbazine for injection. (See WARNINGS.)
- Hepatic necrosis has been reported. (See WARNINGS.)
- Studies have demonstrated this agent to have a carcinogenic and teratogenic effect when used in animals.
- In treatment of each patient, the physician must weigh carefully the possibility of achieving therapeutic benefit against the risk of toxicity.
-
DESCRIPTION
Dacarbazine for Injection, USP is a white to an ivory colored solid which is light sensitive. Each 20 mL vial contains 200 mg of dacarbazine, USP (active ingredient). Each vial also contains anhydrous citric acid and mannitol. Dacarbazine for Injection, USP is reconstituted and administered intravenously (pH 3.0 to 4.0). Dacarbazine for Injection, USP is an anticancer agent. Chemically, Dacarbazine for Injection, USP is 5-(3,3-Dimethyl-1-triazeno) imidazole-4-carboxamide (dacarbazine) with the following structural formula:
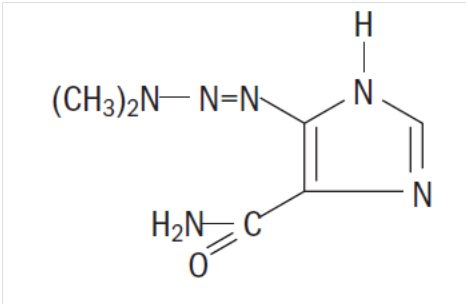
C6H10N6O M.W. 182.19
-
CLINICAL PHARMACOLOGY
After intravenous administration of dacarbazine for injection, the volume of distribution exceeds total body water content suggesting localization in some body tissue, probably the liver. Its disappearance from the plasma is biphasic with initial half-life of 19 minutes and a terminal half-life of 5 hours.1 In a patient with renal and hepatic dysfunctions, the half-lives were lengthened to 55 minutes and 7.2 hours.1 The average cumulative excretion of unchanged dacarbazine in the urine is 40% of the injected dose in 6 hours.1
Dacarbazine is subject to renal tubular secretion rather than glomerular filtration. At therapeutic concentrations dacarbazine is not appreciably bound to human plasma protein.
In man, dacarbazine is extensively degraded. Besides unchanged dacarbazine, 5-aminoimidazole -4 carboxamide (AIC) is a major metabolite of dacarbazine excreted in the urine. AIC is not derived endogenously but from the injected dacarbazine, because the administration of radioactive dacarbazine labeled with 14C in the imidazole portion of the molecule (dacarbazine-2-14C) gives rise to AIC-2-14C.1
Although the exact mechanism of action of dacarbazine for injection is not known, three hypotheses have been offered:
- Inhibition of DNA synthesis by acting as a purine analog
- Action as an alkylating agent
- Interaction with SH groups
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
Hemopoietic depression is the most common toxicity with dacarbazine for injection and involves primarily the leukocytes and platelets, although anemia may sometimes occur. Leukopenia and thrombocytopenia may be severe enough to cause death. The possible bone marrow depression requires careful monitoring of white blood cells, red blood cells, and platelet levels. Hemopoietic toxicity may warrant temporary suspension or cessation of therapy with dacarbazine for injection.
Hepatic toxicity accompanied by hepatic vein thrombosis and hepatocellular necrosis resulting in death, has been reported. The incidence of such reactions has been low; approximately 0.01% of patients treated. This toxicity has been observed mostly when dacarbazine for injection has been administered concomitantly with other anti-neoplastic drugs; however, it has also been reported in some patients treated with dacarbazine for injection alone.
Anaphylaxis can occur following the administration of dacarbazine for injection.
-
PRECAUTIONS
Hospitalization is not always necessary but adequate laboratory study capability must be available. Extravasation of the drug subcutaneously during intravenous administration may result in tissue damage and severe pain. Local pain, burning sensation, and irritation at the site of injection may be relieved by locally applied hot packs.
Carcinogenicity of dacarbazine was studied in rats and mice. Proliferative endocardial lesions, including fibrosarcomas and sarcomas were induced by dacarbazine in rats. In mice, administration of dacarbazine resulted in the induction of angiosarcomas of the spleen.
Pregnancy
Teratogenic Effects, Pregnancy Category C
Dacarbazine for injection has been shown to be teratogenic in rats when given in doses 20 times the human daily dose on day 12 of gestation. Dacarbazine when administered in 10 times the human daily dose to male rats (twice weekly for 9 weeks) did not affect the male libido, although female rats mated to male rats had higher incidence of resorptions than controls. In rabbits, dacarbazine daily dose 7 times the human daily dose given on days 6 to 15 of gestation resulted in fetal skeletal anomalies. There are no adequate and well controlled studies in pregnant women. Dacarbazine for injection should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for tumorigenicity shown for dacarbazine for injection in animal studies, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
-
ADVERSE REACTIONS
Symptoms of anorexia, nausea, and vomiting are the most frequently noted of all toxic reactions. Over 90% of patients are affected with the initial few doses. The vomiting lasts 1 to 12 hours and is incompletely and unpredictably palliated with phenobarbital and/or prochlorperazine. Rarely, intractable nausea and vomiting have necessitated discontinuance of therapy with dacarbazine for injection. Rarely, dacarbazine for injection has caused diarrhea. Some helpful suggestions include restricting the patient's oral intake of food for 4 to 6 hours prior to treatment. The rapid toleration of these symptoms suggests that a central nervous system mechanism may be involved, and usually these symptoms subside after the first 1 or 2 days.
There are a number of minor toxicities that are infrequently noted. Patients have experienced an influenza-like syndrome of fever to 39°C, myalgias and malaise. These symptoms occur usually after large single doses, may last for several days, and they may occur with successive treatments.
Alopecia has been noted as has facial flushing and facial paresthesia. There have been few reports of significant liver or renal function test abnormalities in man. However, these abnormalities have been observed more frequently in animal studies.
Erythematous and urticarial rashes have been observed infrequently after administration of dacarbazine for injection. Rarely, photosensitivity reactions may occur.
To report SUSPECTED ADVERSE REACTIONS, contact Teva Pharmaceuticals USA, Inc. at 1-888-838-2872 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
- OVERDOSAGE
-
DOSAGE AND ADMINISTRATION
Malignant Melanoma
The recommended dosage is 2 to 4.5 mg/kg/day for 10 days. Treatment may be repeated at 4 week intervals.2
An alternate recommended dosage is 250 mg/square meter body surface/day intravenous for 5 days. Treatment may be repeated every 3 weeks.3, 4
Hodgkin's Disease
The recommended dosage of dacarbazine for injection in the treatment of Hodgkin's Disease is 150 mg/square meter body surface/day for 5 days, in combination with other effective drugs. Treatment may be repeated every 4 weeks.5 An alternative recommended dosage is 375 mg/square meter body surface on day 1, in combination with other effective drugs, to be repeated every 15 days.6
Dacarbazine for injection 200 mg/vial is reconstituted with 19.7 mL of sterile water for injection, USP. Dacarbazine for injection 500 mg/vial is reconstituted with 49.25 mL of sterile water for injection, USP. The resulting solution contains 10 mg/mL of dacarbazine having a pH of 3.0 to 4.0. The calculated dose of the resulting solution is drawn into a syringe and administered only intravenously.
The reconstituted solution may be further diluted with 5% dextrose injection or sodium chloride injection and administered as an intravenous infusion.
After reconstitution and prior to use, the solution in the vial may be stored at 4°C for up to 72 hours or at normal room conditions (temperature and light) for up to 8 hours. If the reconstituted solution is further diluted in 5% dextrose injection or sodium chloride injection, the resulting solution may be stored at 4°C for up to 24 hours or at normal room conditions for up to 8 hours.
Procedures for proper handling and disposal of anticancer drugs should be considered. Several guidelines on this subject have been published.7–12 There is no general agreement that all of the procedures recommended in the guidelines are necessary or appropriate.
- HOW SUPPLIED
-
REFERENCES
- Loo, T.J., et al.: Mechanism of action and pharmacology studies with DTIC (NSC-45388). Cancer Treatment Reports 60: 149–152, 1976.
- Nathanson, L., et al.: Characteristics of prognosis and response to an imidazole carboxamide in malignant melanoma. Clinical Pharmacology and Therapeutics 12: 955–962, 1971.
- Costanza, M.E., et al.: Therapy of malignant melanoma with an imidazole carboxamide and bischloroethyl nitrosourea. Cancer 30: 1457–1461, 1972.
- Luce, J.K., et al.: Clinical trials with the antitumor agent 5-(3, 3-dimethyl-1-triazeno) imidazole-4-carboxamide (NSC-45388). Cancer Chemotherapy Reports 54: 119–124, 1970.
- Bonadonna, G., et al.: Combined Chemotherapy (MOPP or ABVD)—radiotherapy approach in advanced Hodgkin's disease. Cancer Treatment Reports 61: 769–777, 1977.
- Santoro, A., and Bonadonna, G.: Prolonged disease-free survival in MOPP-resistant Hodgkin's disease after treatment with adriamycin, bleomycin, vinblastine and dacarbazine (ABVD).
Cancer Chemotherapy Pharmacol. 2: 101–105, 1979. - Recommendations for the Safe Handling of Parenteral Antineoplastic Drugs, NIH Publication No. 83-2621. For sale by the Superintendent of Documents, U.S. Government Printing Office, Washington, DC 20402.
- AMA Council Report, Guidelines for Handling Parenteral Antineoplastics, JAMA, 1985; 253 (11):1590–1592.
- National Study Commission on Cytotoxic Exposure—Recommendations for Handling Cytotoxic Agents. Available from Louis P. Jeffrey, ScD., Chairman, National Study Commission on Cytotoxic Exposure, Massachusetts College of Pharmacy and Allied Health Sciences, 179 Longwood Avenue, Boston, Massachusetts 02115.
- Clinical Oncological Society of Australia, Guidelines and Recommendations for Safe Handling of Antineoplastic Agents. Med. J. Australia, 1983; 1:426–428.
- Jones, RB, et al.: Safe Handling of Chemotherapeutic Agents: A Report from the Mount Sinai Medical Center. CA—A Cancer Journal for Clinicians, 1983; (Sept/Oct) 258–263.
- American Society of Hospital Pharmacists Technical Assistance Bulletin on Handling Cytotoxic and Hazardous Drugs. Am. J. Hosp. Pharm, 1990; 47: 1033–1049.
- OSHA Work-Practice Guidelines for Personnel Dealing with Cytotoxic (Antineoplastic) Drugs. Am. J. Hosp. Pharm, 1986; 43:1193–1204.
Distributed by:
Teva Pharmaceuticals USA, Inc.
Parsippany, NJ 07054Rev. B 2/2020
- Package/Label Display Panel
-
INGREDIENTS AND APPEARANCE
DACARBAZINE
dacarbazine injection, powder, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0703-5075 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DACARBAZINE (UNII: 7GR28W0FJI) (DACARBAZINE - UNII:7GR28W0FJI) DACARBAZINE 200 mg in 20 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) MANNITOL (UNII: 3OWL53L36A) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0703-5075-01 1 in 1 CARTON 08/27/1998 05/31/2024 1 20 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product 2 NDC:0703-5075-03 10 in 1 TRAY 08/27/1998 02/29/2024 2 20 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA075259 08/27/1998 05/31/2024 Labeler - Teva Parenteral Medicines, Inc. (794362533)