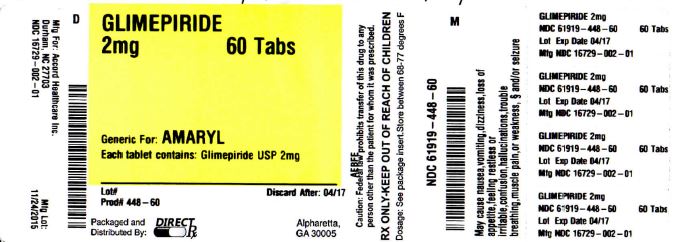
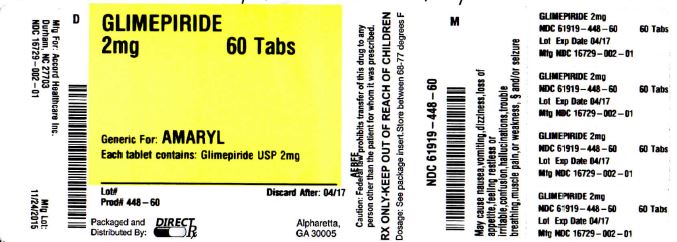
Label: GLIMEPIRIDE tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 61919-448-60 - Packager: DirectRX
- This is a repackaged label.
- Source NDC Code(s): 16729-002
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 24, 2015
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Dosage and Administration
2.1 Recommended Dosing
Glimepiride tablets should be administered with breakfast or the first main meal of the day.
The recommended starting dose of glimepiride tablet is 1 mg or 2 mg once daily. Patients at increased risk for hypoglycemia (e.g., the elderly or patients with renal impairment) should be started on 1 mg once daily [see Warnings and Precautions ( 5.1) and Use in Specific Populations ( 8.5, 8.6)].
After reaching a daily dose of 2 mg, further dose increases can be made in increments of 1 mg or 2 mg based upon the patient’s glycemic response. Uptitration should not occur more frequently than every 1 to 2 weeks. A conservative titration scheme is recommended for patients at increased risk for hypoglycemia [see Warnings and Precautions ( 5.1) and Use in Specific Populations ( 8.5, 8.6)].
The maximum recommended dose is 8 mg once daily.
Patients being transferred to glimepiride from longer half-life sulfonylureas (e.g., chlorpropamide) may have overlapping drug effect for 1 to 2 weeks and should be appropriately monitored for hypoglycemia.
When colesevelam is coadministered with glimepiride, maximum plasma concentration and total exposure to glimepiride is reduced. Therefore, glimepiride should be administered at least 4 hours prior to colesevelam.
-
Dosage Forms and Strengths
Glimepiride tablet is formulated as tablets of:
1 mg tablets (pink coloured, oval shaped, biconvex, uncoated tablets debossed with ‘AHI 1’ on one side and break line on the other)
2 mg tablets (green coloured, oval shaped, biconvex, uncoated tablets debossed with ‘AHI 2’ on one side and break line on the other)
4 mg tablets (blue coloured, oval shaped, biconvex, uncoated tablets debossed with ‘AHI 4’ on one side and break line on the other) -
Contraindications
Glimepiride tablet is contraindicated in patients with a history of a hypersensitivity reaction to:
Glimepiride or any of the product’s ingredients [see Warnings and Precautions ( 5.2)].
Sulfonamide derivatives: Patients who have developed an allergic reaction to sulfonamide derivatives may develop an allergic reaction to glimepiride. Do not use glimepiride in patients who have a history of an allergic reaction to sulfonamide derivatives.
Reported hypersensitivity reactions include cutaneous eruptions with or without pruritus as well as more serious reactions (e.g. anaphylaxis, angioedema, Stevens-Johnson Syndrome, dyspnea) [see Warnings and Precautions ( 5.2) and Adverse Reactions ( 6.2)].
- Warnings and Precautions
- Drug Interactions
- Use in Specific Populations
- Overdosage
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- Package Label
- Indications and Usage
-
Adverse Reactions
The following serious adverse reactions are discussed in more detail below and elsewhere in the labeling:
Hypoglycemia [see Warnings and Precautions ( 5.1) ]
Hemolytic anemia [see Warnings and Precautions ( 5.3) ]In clinical trials, the most common adverse reactions with glimepiride were hypoglycemia, dizziness, asthenia, headache, and nausea.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. Approximately 2,800 patients with type 2 diabetes have been treated with glimepiride in the controlled clinical trials. In these trials, approximately 1,700 patients were treated with glimepiride for at least 1 year.
Table 1 summarizes adverse events, other than hypoglycemia, that were reported in 11 pooled placebo-controlled trials, whether or not considered to be possibly or probably related to study medication. Treatment duration ranged from 13 weeks to 12 months. Terms that are reported represent those that occurred at an incidence of ≥5% among glimepiride-treated patients and more commonly than in patients who received placebo.
Hypoglycemia:
In a randomized, double-blind, placebo-controlled monotherapy trial of 14 weeks duration, patients already on sulfonylurea therapy underwent a 3-week washout period then were randomized to glimepiride 1 mg, 4 mg, 8 mg or placebo. Patients randomized to glimepiride 4 mg or 8 mg underwent forced-titration from an initial dose of 1 mg to these final doses, as tolerated [see Clinical Studies ( 14.1)]. The overall incidence of possible hypoglycemia (defined by the presence of at least one symptom that the investigator believed might be related to hypoglycemia; a concurrent glucose measurement was not required) was 4% for glimepiride 1 mg, 17% for glimepiride 4 mg, 16% for glimepiride 8 mg and 0% for placebo. All of these events were self-treated.
In a randomized, double-blind, placebo-controlled monotherapy trial of 22 weeks duration, patients received a starting dose of either 1 mg glimepiride or placebo daily. The dose of glimepiride was titrated to a target fasting plasma glucose of 90 to 150 mg/dL. Final daily doses of glimepiride were 1, 2, 3, 4, 6 or 8 mg [see Clinical Studies ( 14.1)]. The overall incidence of possible hypoglycemia (as defined above for the 14-week trial) for glimepiride vs. placebo was 19.7% vs. 3.2%. All of these events were self-treated.
Weight gain: glimepiride, like all sulfonylureas, can cause weight gain [see Clinical Studies ( 14.1)].
Allergic Reactions: In clinical trials, allergic reactions, such as pruritus, erythema, urticaria, and morbilliform or maculopapular eruptions, occurred in less than 1% of glimepiride- treated patients. These may resolve despite continued treatment with glimepiride. There are postmarketing reports of more serious allergic reactions (e.g., dyspnea, hypotension, shock) [see Warnings and Precautions ( 5.2)].
Laboratory Tests:
Elevated Serum Alanine Aminotransferase (ALT): ): In 11 pooled placebo-controlled trials of glimepiride, 1.9% of glimepiride-treated patients and 0.8% of placebo-treated patients developed serum ALT greater than 2 times the upper limit of the reference range.6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of glimepiride. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Serious hypersensitivity reactions, including anaphylaxis, angioedema, and Stevens-Johnson Syndrome [see Warnings and Precautions ( 5.2)]
Hemolytic anemia in patients with and without G6PD deficiency [see Warnings and Precautions ( 5.3)]
Impairment of liver function (e.g. with cholestasis and jaundice), as well as hepatitis, which may progress to liver failure.
Porphyria cutanea tarda, photosensitivity reactions and allergic vasculitis
Leukopenia, agranulocytosis, aplastic anemia, and pancytopenia
Thrombocytopenia (including severe cases with platelet count less than 10,000/μL) and thrombocytopenic purpura
Hepatic porphyria reactions and disulfiram-like reactions
Hyponatremia and syndrome of inappropriate antidiuretic hormone secretion (SIADH), most often in patients who are on other medications or who have medical conditions known to cause hyponatremia or increase release of antidiuretic hormone- to report suspected adverse reactions call 1-800-332-1088 1
- 1
- to report suspected adverse reactions call 1-800-332-1088
-
INGREDIENTS AND APPEARANCE
GLIMEPIRIDE
glimepiride tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:61919-448(NDC:16729-002) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLIMEPIRIDE (UNII: 6KY687524K) (GLIMEPIRIDE - UNII:6KY687524K) GLIMEPIRIDE 2 mg Inactive Ingredients Ingredient Name Strength FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) POVIDONE (UNII: FZ989GH94E) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color green Score 2 pieces Shape OVAL Size 10mm Flavor Imprint Code AHI;2 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61919-448-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 11/24/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078181 11/24/2015 Labeler - DirectRX (079254320) Registrant - DirectRX (079254320) Establishment Name Address ID/FEI Business Operations DirectRX 079254320 repack(61919-448)