Label: MITOSOL- mitomycin kit
- NDC Code(s): 49771-002-01, 49771-002-03
- Packager: Mobius Therapeutics LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 15, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use MITOSOL® safely and effectively. See full prescribing information for MITOSOL®.
MITOSOL® (mitomycin for solution) for ophthalmic use
Initial U.S. Approval: 1974INDICATIONS AND USAGE
Mitosol® is an antimetabolite indicated as an adjunct to ab externo glaucoma surgery. (1)
DOSAGE AND ADMINISTRATION
Mitosol® is intended for topical application to the surgical site of glaucoma filtration surgery. It is not intended for intraocular administration. (2)
- •
- Each vial of Mitosol® contains 0.2 mg of mitomycin and mannitol in a 1:2 concentration ratio. To reconstitute, add 1 mL of Sterile Water for Injection, then shake to dissolve. If product does not dissolve immediately, allow to stand at room temperature until the product has dissolved into solution. (2.2)
- •
- Fully saturate sponges provided within the Mitosol® Kit utilizing the entire reconstituted contents of the vial in the manner prescribed in the Instructions for Use. (2.3)
- •
- Apply fully saturated sponges equally to the treatment area, in a single layer, with the use of a surgical forceps. Keep the sponges on the treatment area for two (2) minutes, then remove and return to the Mitosol® Tray for defined disposal. (2.3)
DOSAGE FORMS AND STRENGTHS
Each vial contains a sterile lyophilized mixture of 0.2 mg mitomycin and 0.4 mg mannitol; when reconstituted with Sterile Water for Injection, the solution contains 0.2 mg/mL mitomycin. (3)
CONTRAINDICATIONS
- •
- Hypersensitivity to mitomycin. (4.1)
WARNINGS AND PRECAUTIONS
- •
- Cell Death: Mitomycin is cytotoxic. Use of mitomycin in concentrations higher than 0.2 mg/mL or use for longer than 2 minutes may lead to unintended corneal and/or scleral damage including thinning or perforation. Direct contact with the corneal endothelium will result in cell death. (5.1)
- •
- Hypotony: The use of mitomycin has been associated with an increased incidence of post-operative hypotony. (5.2)
- •
- Cataract Development: Use in phakic patients has been correlated to a higher incidence of lenticular change and cataract formation. (5.3)
- •
- Embryo-Fetal Toxicity: Can cause fetal harm. Advise of potential risk to a fetus. Verify pregnancy status in females of reproductive potential prior to use. (5.4, 8.1, 8.3)
ADVERSE REACTIONS
The most frequent adverse reactions to Mitosol® occur locally and include hypotony, hypotony maculopathy, blebitis, endophthalmitis, vascular reactions, corneal reactions, and cataract. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Mobius Therapeutics LLC at 1-877-393-6486 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 4/2021
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
2.2 Method of Reconstitution
2.3 Method of Use
2.4 Stability
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Hypersensitivity
5 WARNINGS AND PRECAUTIONS
5.1 Cell Death
5.2 Hypotony
5.3 Cataract Formation
5.4 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
6.1 Ophthalmic Adverse Reactions
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
Mitosol® is intended for topical application to the surgical site of glaucoma filtration surgery. Mitosol® is a cytotoxic drug. It is not intended for intraocular administration. If intraocular administration occurs, cell death leading to corneal infarction, retinal infarction, and ciliary body atrophy may result. Verify pregnancy status in females of reproductive potential prior to using Mitosol®.
2.2 Method of Reconstitution
Each vial of Mitosol® contains 0.2 mg of mitomycin and mannitol in a 1:2 concentration ratio. To reconstitute, add 1 mL of Sterile Water for Injection, then shake to dissolve. If product does not dissolve immediately, allow to stand at room temperature until the product dissolves into solution.
2.3 Method of Use
Sponges provided within the Mitosol® Kit should be fully saturated with the entire reconstituted contents in the manner prescribed in the Instructions for Use. A treatment area approximating 10mm x 6mm +/- 2mm should be treated with the Mitosol®. Apply fully saturated sponges equally to the treatment area, in a single layer, with the use of a surgical forceps. Keep the sponges on the treatment area for two (2) minutes, then remove and return to the Mitosol® Tray for defined disposal in the Chemotherapy Waste Bag provided.
2.4 Stability
Lyophilized Mitosol® stored at 20°C to 25°C (68°F to 77°F) is stable for the shelf life indicated on the package. Avoid excessive heat. Protect from light.
Reconstituted with 1 mL of Sterile Water for Injection at a concentration of 0.2 mg/mL, mitomycin is stable for one (1) hour at room temperature.
-
3 DOSAGE FORMS AND STRENGTHS
Mitosol® is a sterile lyophilized mixture of mitomycin and mannitol, which, when reconstituted with Sterile Water for Injection, provides a solution for application in glaucoma filtration surgery. Mitosol® is supplied in vials containing 0.2 mg of mitomycin. Each vial also contains mannitol 0.4 mg, at a 1:2 ratio of mitomycin to mannitol. Each mL of reconstituted solution contains 0.2 mg mitomycin and has a pH between 5.0 and 8.0.
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Cell Death
Mitomycin is cytotoxic. Use of mitomycin in concentrations higher than 0.2 mg/mL or use for longer than 2 minutes may lead to unintended corneal and/or scleral damage including thinning or perforation. Direct contact with the corneal endothelium will result in cell death.
5.2 Hypotony
The use of mitomycin has been associated with an increased incidence of post-operative hypotony.
5.3 Cataract Formation
Use in phakic patients has been correlated to a higher incidence of lenticular change and cataract formation.
5.4 Embryo-Fetal Toxicity
Based on findings in animals and mechanism of action, Mitosol® can cause fetal harm when administered to a pregnant woman. In animal reproduction studies, parenteral administration of mitomycin resulted in teratogenicity [see Use in Specific Populations (8.1, 8.3) and Clinical Pharmacology (12.1)].
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- •
- Cell Death [see Warnings and Precautions (5.1)]
- •
- Hypotony [see Warnings and Precautions (5.2)]
- •
- Cataract Formation [see Warnings and Precautions (5.3)]
6.1 Ophthalmic Adverse Reactions
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. The most frequent adverse reactions to Mitosol® occur locally, as an extension of the pharmacological activity of the drug. These reactions include:
Blebitis: bleb ulceration, chronic bleb leak, encapsulated/cystic bleb, bleb-related infection, wound dehiscence, conjunctival necrosis, thin-walled bleb
Cornea: corneal endothelial damage, epithelial defect, anterior synechiae, superficial punctuate keratitis, Descemet's detachment, induced astigmatism
Endophthalmitis
Hypotony: choroidal reactions (choroidal detachment, choroidal effusion, serous choroidal detachment, suprachoroidal hemorrhage, hypotony maculopathy, presence of supraciliochoroidal fluid, hypoechogenic suprachoroidal effusion)
Inflammation: iritis, fibrin reaction
Lens: cataract development, cataract progression, capsule opacification, capsular constriction and/or capsulotomy rupture, posterior synechiae
Retina: retinal pigment epithelial tear, retinal detachment (serous and rhegatogenous)
Scleritis: wound dehiscence
Vascular: hyphema, central retinal vein occlusion, hemiretinal vein occlusion, retinal hemorrhage, vitreal hemorrhage and blood clot, subconjunctival hemorrhage, disk hemorrhage
Additional Reactions: macular edema, sclera thinning or ulceration, intraocular lens capture, disk swelling, malignant glaucoma, lacrimal drainage system obstruction, ciliary block, corneal vascularization, visual acuity decrease, cystic conjunctival degeneration, upper eyelid retraction, dislocated implants, severe loss of vision.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on findings in animals and mechanism of action [see Clinical Pharmacology (12.1)], Mitosol® can cause fetal harm when administered to a pregnant woman. There are no available data on Mitosol® use in pregnant women to inform the drug-associated risk. In animal reproduction studies, parenteral administration of mitomycin resulted in teratogenicity (see Data). Advise pregnant women of the potential risk to a fetus.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% - 4% and 15% - 20%, respectively.
8.2 Lactation
Risk Summary
There are no data on the presence of mitomycin in human milk, the effects on the breastfed child, or the effects on milk production. Because of the potential for serious adverse reactions in a breastfed child, advise women not to breastfeed during and for 1 week following administration of Mitosol®.
8.3 Females and Males of Reproductive Potential
Mitosol® can cause fetal harm when administered to pregnant women [see Use in Specific Populations (8.1)].
-
11 DESCRIPTION
Mitomycin is an antibiotic isolated from the broth of Streptomyces verticillus Yingtanensis which has been shown to have antimetabolic activity.
Mitomycin is a blue-violet crystalline powder with the molecular formula of C15H18N4O5 and a molecular weight of 334.33. Its chemical name is 7-amino-9α-methoxymitosane and it has the following structural formula:
Mitosol® is a sterile lyophilized mixture of mitomycin and mannitol, which, when reconstituted with Sterile Water for Injection, provides a solution for application in glaucoma filtration surgery. Mitosol® is supplied in vials containing 0.2 mg of mitomycin. Each vial also contains mannitol 0.4 mg, at a 1:2 ratio of mitomycin to mannitol. Each mL of reconstituted solution contains 0.2 mg mitomycin and has a pH between 5.0 and 8.0.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Mitosol® inhibits the synthesis of deoxyribonucleic acid (DNA). The guanine and cytosine content correlates with the degree of mitomycin-induced cross-linking. Cellular RNA and protein synthesis may also be suppressed.
12.3 Pharmacokinetics
Absorption
The systemic exposure of mitomycin following ocular administration of Mitosol® in humans is unknown. Based on a comparison of the proposed dose of up to 0.2 mg to intravenous (IV) doses of mitomycin used clinically for treatment of oncologic indications (up to 20 mg/m2), systemic concentrations in humans upon ocular administration are expected to be multiple orders of magnitude lower than those achieved by IV administration.
Elimination
Metabolism
In humans, mitomycin is cleared from ophthalmic tissue after intraoperative topical application and irrigation, as metabolism occurs in other affected tissues. Systemic clearance is affected primarily by metabolism in the liver. The rate of clearance is inversely proportional to the maximal serum concentration because of saturation of the degradative pathways.
Excretion
Approximately 10% of an injectable dose of mitomycin is excreted unchanged in the urine. Since metabolic pathways are saturated at relatively low doses, the percent of a dose excreted in urine increases.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Adequate long-term studies in animals to evaluate carcinogenic potential have not been conducted with Mitosol®. Intravenous administration of mitomycin has been found to be carcinogenic in rats and mice. At doses approximating the recommended clinical injectable dose in humans, mitomycin produces a greater than 100 percent increase in tumor incidence in male Sprague-Dawley rats, and a greater than 50 percent increase in tumor incidence in female Swiss mice.
The effect of Mitosol® on fertility is unknown.
-
14 CLINICAL STUDIES
In placebo-controlled studies reported in the medical literature, mitomycin reduced intraocular pressure (IOP) by 3 mmHg in patients with open-angle glaucoma when used as an adjunct to ab externo glaucoma surgery by Month 12.
In studies with a historical control reported in the medical literature, mitomycin reduced intraocular pressure (IOP) by 5 mmHg in patients with open-angle glaucoma when used as an adjunct to ab externo glaucoma surgery by Month 12.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
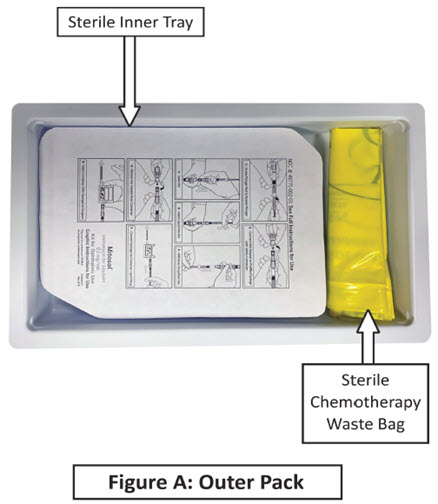
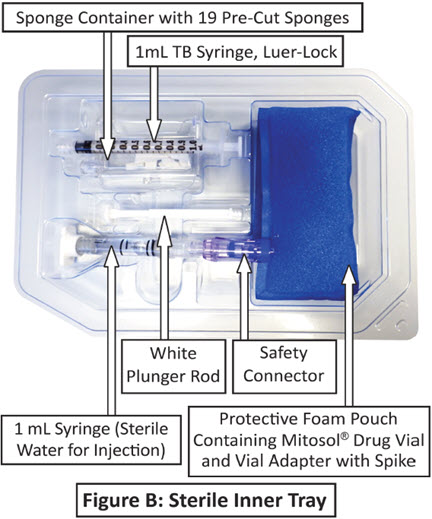
Mitosol® (mitomycin for solution) is available in a kit containing:
One Vial containing 0.2 mg mitomycin
One 1 mL syringe (Sterile Water For Injection) with Safety Connector
One Plunger Rod
One Vial Adapter with Spike
One 1 mL TB Syringe, Luer Lock
One Sponge Container
Six 3 mm Absorbent Sponges
Six 6 mm Absorbent Sponges
Six Half Moon Sponges
One Instrument Wedge Sponge
One Protective Foam Pouch
One Chemotherapy Waste Bag
One Label, MMC (mitomycin)
Three kits are supplied in each carton (NDC 49771-002-03).
-
17 PATIENT COUNSELING INFORMATION
- •
- Instruct patients to discuss with their physician if they are pregnant or if they might become pregnant [see Use in Specific Populations (8.1)].
- •
- Instruct patients to discuss with their physician if they have demonstrated a hypersensitivity to mitomycin in the past [see Contraindications (4.1)].
- •
- Nursing mothers should be advised that it is not known if Mitosol® is excreted in human milk. Because of the potential for serious adverse reactions in a breastfed child, advise women not to breastfeed during and for 1 week following administration of Mitosol®[see Use in Specific Populations (8.2)].
- •
- Patients should be advised of the toxicity of Mitosol® and potential complications.
Manufactured for:
Mobius Therapeutics, LLC
1000 Executive Parkway
Suite 224
St. Louis, MO 63141 -
Mitosol®
(mitomycin for solution)
0.2 mg/vial
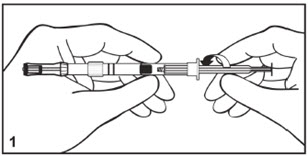
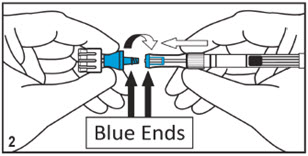
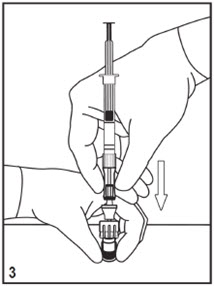
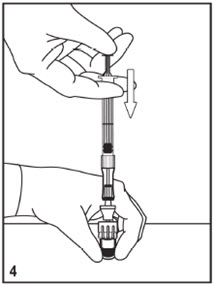
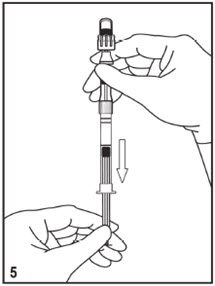
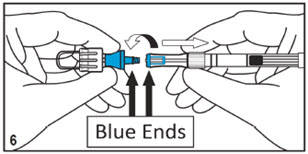
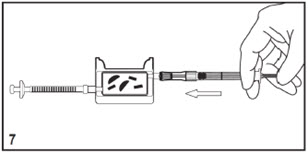
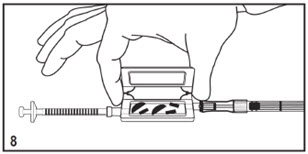
Kit for Ophthalmic UseRead INSTRUCTIONS FOR USE Before Proceeding
Instructions for Use
DISPOSE OF CHEMOTHERAPY WASTE BAG AND ITS CONTENTS AS CHEMOTHERAPY WASTE
US Patents #7,806,265, #8,186,511, #D685,962, #D685,963, #9,205,075, #9,539,241 and #9,649,428; other international patents issued and pending.
A4807998-2
Rev. 07/20 -
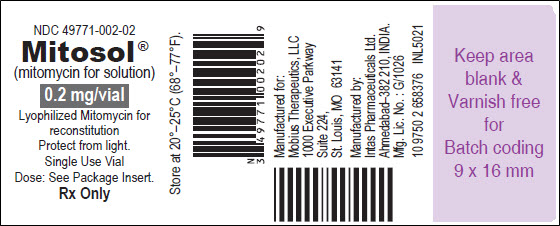
PRINCIPAL DISPLAY PANEL - VIAL LABEL
NDC 49771-002-02
Mitosol®
(mitomycin for solution)0.2 mg/vial
Lyophilized Mitomycin for
reconstitution
Protect from light.
Single Use Vial
Dose: See Package Insert.Rx Only
Store at 20°-25°C (68°-77°F).
Manufactured for:
Mobius Therapeutics, LLC
1000 Executive Parkway
Suite 224
St. Louis, MO 63141Manufactured by:
Intas Pharmaceuticals Ltd.
Ahmedabad-382 210, INDIA.Mfg. Lic. No.: G/1026
10 9750 2 658376 INL5021
-
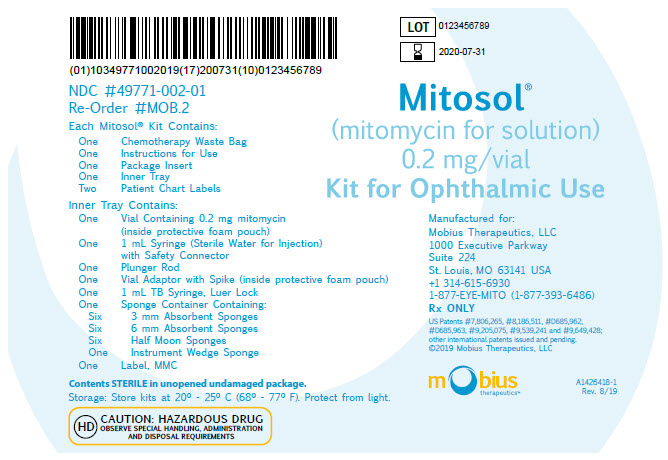
PRINCIPAL DISPLAY PANEL - OUTER KIT PACKAGE
Mitosol®
(mitomycin for solution)
0.2 mg/vialKit for Ophthalmic Use
Manufactured for:
Mobius Therapeutics, LLC
1000 Executive Parkway
Suite 224
St. Louis, MO 63141 USA
+1 314-615-6930
1-877-EYE-MITO (1-877-393-6486)Rx ONLY
US Patents #7,806,265, #8,186,511, #D685,962,
#D685,963, #9,205,075, #9,539,241 and #9,649,428;
other international patents issued and pending.©2019 Mobius Therapeutics, LLC
mobius
therapeutics™A1426418-1
Rev. 8/19NDC #49771-002-01
Re-Order #MOB.2Each Mitosol® Kit Contains:
One Chemotherapy Waste Bag
One Instructions for Use
One Package Insert
One Inner Tray
Two Patient Chart LabelsInner Tray Contains:
One Vial Containing 0.2 mg mitomycin
(inside protective foam pouch)
One 1 mL Syringe (Sterile Water for Injection)
with Safety Connector
One Plunger Rod
One Vial Adaptor with Spike (inside protective foam pouch)
One 1 mL TB Syringe, Luer Lock
One Sponge Container Containing:
Six 3 mm Absorbent Sponges
Six 6 mm Absorbent Sponges
Six Half Moon Sponges
One Instrument Wedge Sponge
One Label, MMCContents STERILE in unopened undamaged package.
Storage: Store kits at 20° - 25° C (68° - 77° F). Protect from light.
HD CAUTION: HAZARDOUS DRUG
OBSERVE SPECIAL HANDLING, ADMINISTRATION
AND DISPOSAL REQUIREMENTS -
INGREDIENTS AND APPEARANCE
MITOSOL
mitomycin kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:49771-002 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49771-002-03 3 in 1 CARTON 02/08/2012 1 NDC:49771-002-01 1 in 1 TRAY; Type 0: Not a Combination Product Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, SINGLE-USE 1 mL Part 2 1 SYRINGE, GLASS 1 mL Part 1 of 2 MITOSOL
mitomycin injection, powder, lyophilized, for solutionProduct Information Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Mitomycin (UNII: 50SG953SK6) (Mitomycin - UNII:50SG953SK6) Mitomycin 0.2 mg in 1 mL Inactive Ingredients Ingredient Name Strength mannitol (UNII: 3OWL53L36A) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022572 02/08/2012 Part 2 of 2 STERILE WATER
water injection, solutionProduct Information Route of Administration OPHTHALMIC Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 mL in 1 SYRINGE, GLASS; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022572 02/08/2012 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022572 02/08/2012 Labeler - Mobius Therapeutics LLC (805642118)