TRANZAREL- lidocaine gel
Taleos Pharma
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
TRANZAREL (Lidocaine) 4% Pain Relieving Gel
DESCRIPTION
TRANZAREL 4% is comprised of a topical anesthetic that is indicated for relief of minor pain.
Lidocaine is chemically designated as acetamide, 2-(diethylamino)-N-(2,6-dimethylphenyl), and has the following structure:
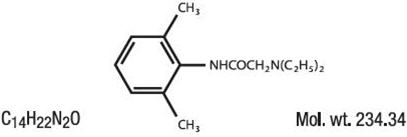
Each gram contains 40 mg of lidocaine in an aqueous gel base. It also contains the following inactive ingredients: Aloe Barbadensis Leaf Juice, Calendula Officinalis Flower Extract, Camellia Sinensis (Green Tea) Leaf Extract, Carbomer, Chamomilla Recutita (Matricaria) Flower Extract, Diazolidinyl Urea, Disodium EDTA, Glycerin, Menthol, Methylparaben, Propylene Glycol, Propylparaben, Purified Water, SD Alcohol 40-2, Symphytum Officinale (Comfrey) Leaf Extract, Triethanolamine.
CLINICAL PHARMACOLOGY
Pharmacodynamics
Lidocaine is an amide-type local anesthetic agent and is suggested to stabilize the neuronal membrane by inhibiting the ionic fluxes required for the initiation and conduction of impulses, thereby producing a localized analgesic effect.
Pharmacokinetics
Lidocaine may be absorbed following topical administration to mucous membranes, its rate and extent of absorption depending upon the specific site of application, duration of exposure, concentration, and total dosage. In general, the rate of absorption of local anesthetic agents following topical application occurs most rapidly after intratracheal administration. Lidocaine is also well-absorbed from the gastrointestinal tract, but little intact drug appears in the circulation because of biotransformation in the liver.
Lidocaine is metabolized rapidly by the liver, and metabolites and unchanged drug are excreted by the kidneys. Biotransformation includes oxidative N-dealkylation, ring hydroxylation, cleavage of the amide linkage, and conjugation. N-dealkylation, a major pathway of biotransformation, yields the metabolites monoethylglycinexylidide and glycinexylidide. The pharmacological/toxicological actions of these metabolites are, similar to but less potent than, those of lidocaine. Approximately 90% of lidocaine administered is excreted in the form of various metabolites, and less than 10% is excreted unchanged. The primary metabolite in urine is a conjugate of 4-hydroxy-2, 6-dimethylaniline. The plasma binding of lidocaine is dependent on drug concentration, and the fraction bound decreases with increasing concentration. At concentrations of 1 to 4 g of free base per mL, 60 to 80 percent of lidocaine is protein bound. Binding is also dependent on the plasma concentration of the alpha-1-acid glycoprotein. Lidocaine crosses the blood-brain and placental barriers, presumably by passive diffusion.
Studies of lidocaine metabolism following intravenous bolus injections have shown that the elimination half-life of this agent is typically 1.5 to 2 hours. Because of the rapid rate at which lidocaine is metabolized, any condition that affects liver function may alter lidocaine kinetics. The half-life may be prolonged two-fold or more in patients with liver dysfunction. Renal dysfunction does not affect lidocaine kinetics but may increase the accumulation of metabolites. Factors such as acidosis and the use of CNS stimulants and depressants affect the CNS levels of lidocaine required to produce overt systemic effects. Objective adverse manifestations become increasingly apparent with increasing venous plasma levels above 6 g free base per mL. In the rhesus monkey, arterial blood levels of 18-21 g/mL have been shown to be threshold for convulsive activity.
INDICATION AND USAGE
TRANZAREL 4% is comprised of a topical anesthetic that is indicated for relief of minor pain.
CONTRAINDICATIONS
TRANZAREL is contraindicated in patients with a history of sensitivity to any of its ingredients or adverse reactions to lidocaine or amide anesthetics. If excessive irritation and significant worsening occur, discontinue use and seek the advice of your physician.
WARNINGS
For external use only. Do not use in eyes.
Keep out of reach of children.
Do not use in large quantities, particularly over raw surfaces or blistered areas. If swallowed, get medical help or contact Poison Control Center right away.
PRECAUTIONS
General
Lidocaine should be used cautiously in those with impaired liver function, as well as the very ill or very elderly and those with significant liver disease. Product should be used with caution in patients receiving antiarrhythmic drugs of Class I since the adverse effects are additive and generally synergistic.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies of lidocaine in animals to evaluate the carcinogenic and mutagenic potential of the effect on fertility have not been conducted.
Pregnancy
Teratogenic Effects
Reproduction studies have been performed for lidocaine in rats at doses up to 6.6 times the human dose and have revealed no evidence of harm to the fetus caused by lidocaine. There are, however, no adequate and well-controlled studies in pregnant women. Since animal reproduction studies are not always predictive of human response, general consideration should be given to this fact before administering lidocaine to women of childbearing potential, especially during early pregnancy.
ADVERSE REACTIONS
During or immediately following application of TRANZAREL, there may be transient stinging or burning from open areas of skin, or transient blanching (lightening), or erythema (redness) of the skin.
Allergic Reactions
Allergic and anaphylactoid reactions associated with lidocaine, although rare, can occur. Such reactions are characterized by angioedema, bronchospasm, dermatitis, dyspnea, hypersensitivity, laryngospasm, pruritus, shock, and urticaria. If they occur, they should be managed by conventional means.
Systemic (Dose-Related) Reactions
Systemic adverse reactions of lidocaine are similar in nature to those observed with other amide local anesthetic agents, including CNS excitation and/or depression (light headedness, nervousness, apprehension, euphoria, confusion, dizziness, drowsiness, tinnitus, blurred or double vision, vomiting, sensations of heat, cold or numbness, twitching, tremors, convulsions, unconsciousness, respiratory depression and arrest). Excitatory CNS reactions may be brief or not occur at all, in which case the first manifestation may be drowsiness merging into unconsciousness. Cardiovascular manifestations may include bradycardia, hypotension and cardiovascular collapse leading to arrest.
DOSAGE AND ADMINISTRATION
Apply TRANZAREL to affected area(s) of pain three to four times a day or as directed by a physician. TRANZAREL should only be applied to intact skin. The cap and foil should be removed from the tube prior to use.
To open tube: Turn cap, push down, turn and lift off.
To close tube: Turn on cap until it locks into place.
May be applied under occlusive dressing. Consult with your physician first.
HOW SUPPLIED
TRANZAREL (Lidocaine) 4% Pain Relieving Gel is available as the following:
4 fl. oz. (120 mL) laminate tube with a child-resistant cap, NDC 70877-2324-4
| TRANZAREL
lidocaine gel |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Labeler - Taleos Pharma (080318529) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| TALEOS PHARMA CORPORATION | 080318529 | manufacture(70877-2324) | |
