Label: YERVOY- ipilimumab injection
- NDC Code(s): 0003-2327-11, 0003-2328-22
- Packager: E.R. Squibb & Sons, L.L.C.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated February 15, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use YERVOY safely and effectively. See full prescribing information for YERVOY.
YERVOY® (ipilimumab) injection, for intravenous use
Initial U.S. Approval: 2011INDICATIONS AND USAGE
YERVOY is a human cytotoxic T-lymphocyte antigen 4 (CTLA-4)-blocking antibody indicated for:
Melanoma
- •
- Treatment of unresectable or metastatic melanoma in adults and pediatric patients 12 years and older as a single agent or in combination with nivolumab. (1.1)
- •
- Adjuvant treatment of adult patients with cutaneous melanoma with pathologic involvement of regional lymph nodes of more than 1 mm who have undergone complete resection, including total lymphadenectomy. (1.2)
Renal Cell Carcinoma (RCC)
- •
- Treatment of adult patients with intermediate or poor risk advanced renal cell carcinoma, as first-line treatment in combination with nivolumab. (1.3)
Colorectal Cancer
- •
- Treatment of adult and pediatric patients 12 years and older with microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) metastatic colorectal cancer that has progressed following treatment with a fluoropyrimidine, oxaliplatin, and irinotecan, in combination with nivolumab. This indication is approved under accelerated approval based on overall response rate and duration of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials. (1.4)
Hepatocellular Carcinoma
- •
- Treatment of adult patients with hepatocellular carcinoma who have been previously treated with sorafenib, in combination with nivolumab. This indication is approved under accelerated approval based on overall response rate and duration of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials. (1.5)
Non-Small Cell Lung Cancer (NSCLC)
- •
- Treatment of adult patients with metastatic non-small cell lung cancer expressing PD-L1 (≥1%) as determined by an FDA-approved test, with no EGFR or ALK genomic tumor aberrations, as first-line treatment in combination with nivolumab. (1.6)
- •
- Treatment of adult patients with metastatic or recurrent non-small cell lung cancer with no EGFR or ALK genomic tumor aberrations as first-line treatment, in combination with nivolumab and 2 cycles of platinum-doublet chemotherapy. (1.6)
Malignant Pleural Mesothelioma
- •
- Treatment of adult patients with unresectable malignant pleural mesothelioma, as first-line treatment in combination with nivolumab. (1.7)
Esophageal Cancer
- •
- Treatment of adult patients with unresectable advanced or metastatic esophageal squamous cell carcinoma, as first line treatment in combination with nivolumab. (1.8)
DOSAGE AND ADMINISTRATION
- •
- Administer by intravenous infusion after dilution based upon recommended infusion rate for each indication. (2)
- •
-
Unresectable or Metastatic Melanoma:
- ∘
- YERVOY 3 mg/kg every 3 weeks for a maximum of 4 doses. (2.2)
- ∘
- YERVOY 3 mg/kg immediately following nivolumab 1 mg/kg on the same day, every 3 weeks for 4 doses. After completing 4 doses of the combination, administer nivolumab as a single agent as recommended in the Full Prescribing Information for nivolumab. (2.2)
- •
- Adjuvant Treatment of Melanoma: YERVOY 10 mg/kg every 3 weeks for 4 doses, followed by 10 mg/kg every 12 weeks for up to 3 years. (2.2)
- •
- Advanced Renal Cell Carcinoma: YERVOY 1 mg/kg immediately following nivolumab 3 mg/kg on the same day, every 3 weeks for 4 doses. After completing 4 doses of the combination, administer nivolumab as a single agent as recommended in Full Prescribing Information for nivolumab. (2.2)
- •
- Microsatellite Instability-High (MSI-H) or Mismatch Repair Deficient (dMMR) Metastatic Colorectal Cancer: YERVOY 1 mg/kg intravenously over 30 minutes immediately following nivolumab 3 mg/kg intravenously over 30 minutes on the same day, every 3 weeks for 4 doses. After completing 4 doses of the combination, administer nivolumab as a single agent as recommended in Full Prescribing Information for nivolumab. (2.2)
- •
- Hepatocellular Carcinoma: YERVOY 3 mg/kg intravenously over 30 minutes immediately following nivolumab 1 mg/kg intravenously over 30 minutes on the same day, every 3 weeks for 4 doses. After completing 4 doses of the combination, administer nivolumab as a single agent as recommended in Full Prescribing Information for nivolumab. (2.2)
- •
- Metastatic non-small cell lung cancer:
- •
- Malignant pleural mesothelioma: YERVOY 1 mg/kg every 6 weeks with nivolumab 360 mg every 3 weeks. (2.2)
- •
- Esophageal squamous cell carcinoma: YERVOY 1 mg/kg every 6 weeks with nivolumab 3 mg/kg every 2 weeks or 360 mg every 3 weeks. (2.2)
- •
- See full Prescribing Information for preparation and administration instructions and dosage modifications for adverse reactions.
DOSAGE FORMS AND STRENGTHS
- •
- Injection: 50 mg/10 mL (5 mg/mL) and 200 mg/40 mL (5 mg/mL) in a single-dose vial. (3)
CONTRAINDICATIONS
- •
- None. (4)
WARNINGS AND PRECAUTIONS
- •
- Severe and Fatal Immune-Mediated Adverse Reactions: Immune-mediated adverse reactions (IMAR) can occur in any organ system or tissue, including the following: immune-mediated colitis, immune-mediated hepatitis, immune-mediated dermatologic adverse reactions, immune-mediated endocrinopathies, immune-mediated pneumonitis, and immune-mediated nephritis with renal dysfunction, and can occur at any time during treatment or after discontinuation. Monitor for symptoms and signs that may be clinical manifestations of IMAR. Evaluate clinical chemistries including liver enzymes, creatinine, adrenocorticotropic hormone level and thyroid function at baseline and before each dose. In general, withhold YERVOY for severe (grade 3) and permanently discontinue for life-threatening (grade 4) immune-mediated adverse reactions. See Full Prescribing Information for additional dosage modifications. (2.3, 5.1)
- •
- Infusion-Related Reactions: Discontinue for severe and life-threatening infusion-related reactions. Interrupt or slow the rate of infusion in patients with mild or moderate infusion-related reactions. (2.3, 5.2)
- •
- Complications of allogeneic HSCT: Fatal and other serious complications can occur in patients who receive allogeneic HSCT before or after being treated with YERVOY. (5.3)
- •
- Embryo-Fetal Toxicity: Can cause fetal harm. Advise of potential risk to a fetus and use of effective contraception. (5.4, 8.1, 8.3)
ADVERSE REACTIONS
Most common adverse reactions (≥5%) with YERVOY as a single agent are fatigue, diarrhea, pruritus, rash, and colitis. Additional common adverse reactions at the 10 mg/kg dose (≥5%) include nausea, vomiting, headache, weight loss, pyrexia, decreased appetite, and insomnia. (6.1)
Most common adverse reactions (≥20%) with YERVOY in combination with nivolumab are fatigue, diarrhea, rash, pruritus, nausea, musculoskeletal pain, pyrexia, cough, decreased appetite, vomiting, abdominal pain, dyspnea, upper respiratory tract infection, arthralgia, headache, hypothyroidism, constipation, decreased weight, and dizziness. (6.1)
Most common adverse reactions (≥20%) with YERVOY in combination with nivolumab and platinum-doublet chemotherapy are fatigue, musculoskeletal pain, nausea, diarrhea, rash, decreased appetite, constipation, and pruritus. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Bristol-Myers Squibb at 1-800-721-5072 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 2/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Unresectable or Metastatic Melanoma
1.2 Adjuvant Treatment of Melanoma
1.3 Advanced Renal Cell Carcinoma
1.4 Microsatellite Instability-High or Mismatch Repair Deficient Metastatic Colorectal Cancer
1.5 Hepatocellular Carcinoma
1.6 Metastatic Non-Small Cell Lung Cancer
1.7 Malignant Pleural Mesothelioma
1.8 Esophageal Cancer
2 DOSAGE AND ADMINISTRATION
2.1 Patient Selection
2.2 Recommended Dosage
2.3 Recommended Dosage Modifications for Adverse Reactions
2.4 Preparation and Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Severe and Fatal Immune-Mediated Adverse Reactions
5.2 Infusion-Related Reactions
5.3 Complications of Allogeneic Hematopoietic Stem Cell Transplant after YERVOY
5.4 Embryo-Fetal Toxicity
5.5 Risks Associated When Administered in Combination with Nivolumab
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Immunogenicity
6.3 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Unresectable or Metastatic Melanoma
14.2 Adjuvant Treatment of Melanoma
14.3 Advanced Renal Cell Carcinoma
14.4 Microsatellite Instability-High or Mismatch Repair Deficient Metastatic Colorectal Cancer
14.5 Hepatocellular Carcinoma
14.6 Metastatic Non-Small Cell Lung Cancer
14.7 Malignant Pleural Mesothelioma
14.8 Esophageal Cancer
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Unresectable or Metastatic Melanoma
YERVOY, as a single agent or in combination with nivolumab, is indicated for the treatment of unresectable or metastatic melanoma in adult and pediatric patients 12 years and older.
1.2 Adjuvant Treatment of Melanoma
YERVOY is indicated for the adjuvant treatment of adult patients with cutaneous melanoma with pathologic involvement of regional lymph nodes of more than 1 mm who have undergone complete resection, including total lymphadenectomy.
1.3 Advanced Renal Cell Carcinoma
YERVOY, in combination with nivolumab, is indicated for the first-line treatment of adult patients with intermediate or poor risk advanced renal cell carcinoma (RCC).
1.4 Microsatellite Instability-High or Mismatch Repair Deficient Metastatic Colorectal Cancer
YERVOY, in combination with nivolumab, is indicated for the treatment of adult and pediatric patients 12 years and older with microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) metastatic colorectal cancer (mCRC) that has progressed following treatment with a fluoropyrimidine, oxaliplatin, and irinotecan.
This indication is approved under accelerated approval based on overall response rate and duration of response [see Clinical Studies (14.4)]. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
1.5 Hepatocellular Carcinoma
YERVOY, in combination with nivolumab, is indicated for the treatment of adult patients with hepatocellular carcinoma (HCC) who have been previously treated with sorafenib. This indication is approved under accelerated approval based on overall response rate and duration of response [see Clinical Studies (14.5)]. Continued approval for this indication may be contingent upon verification and description of clinical benefit in the confirmatory trials.
1.6 Metastatic Non-Small Cell Lung Cancer
YERVOY, in combination with nivolumab, is indicated for the first-line treatment of adult patients with metastatic non-small cell lung cancer (NSCLC) whose tumors express PD-L1 (≥1%) as determined by an FDA-approved test [see Dosage and Administration (2.1)], with no EGFR or ALK genomic tumor aberrations.
YERVOY, in combination with nivolumab and 2 cycles of platinum-doublet chemotherapy, is indicated for the first-line treatment of adult patients with metastatic or recurrent NSCLC, with no EGFR or ALK genomic tumor aberrations.
-
2 DOSAGE AND ADMINISTRATION
2.1 Patient Selection
Select patients with metastatic NSCLC for treatment with YERVOY in combination with nivolumab based on PD-L1 expression [see Clinical Studies (14.6)].
Information on FDA-approved tests for the determination of PD-L1 expression in NSCLC is available at: http://www.fda.gov/CompanionDiagnostics.
2.2 Recommended Dosage
The recommended dosages of YERVOY as a single agent are presented in Table 1.
Table 1: Recommended Dosages for YERVOY as a Single Agent Indication
Recommended YERVOY Dosage
Duration of Therapy
Unresectable or metastatic melanoma
3 mg/kg every 3 weeks
30-minute intravenous infusion
Maximum of 4 doses
Adjuvant treatment of melanoma
10 mg/kg every 3 weeks
followed by 10 mg/kg every 12 weeks
(90-minute intravenous infusion)
Every 3 weeks up to a maximum of 4 doses
Every 12 weeks for up to 3 years
The recommended dosages of YERVOY in combination with other therapeutic agents are presented in Table 2. Refer to the respective Prescribing Information for each therapeutic agent administered in combination with YERVOY for recommended dosage information, as appropriate.
Table 2: Recommended Dosages of YERVOY in Combination with Other Therapeutic Agents* * Refer to the Prescribing Information for the agents administered in combination with YERVOY for recommended dosing information, as appropriate.
† Refer to the Prescribing Information for nivolumab for dosage information after completing use in combination with YERVOY.
‡ 30-minute intravenous infusion on the same day.Indication
Recommended YERVOY Dosage
Duration of Therapy
Unresectable or metastatic melanoma
3 mg/kg every 3 weeks‡
with nivolumab 1 mg/kg‡
In combination with nivolumab for a maximum of 4 doses or until unacceptable toxicity, whichever occurs earlier.
After completing 4 doses of combination therapy, administer nivolumab as a single agent until disease progression or unacceptable toxicity.†
Advanced renal cell carcinoma
1 mg/kg every 3 weeks‡
with nivolumab 3 mg/kg‡
In combination with nivolumab
for a maximum of 4 doses.After completing 4 doses of combination therapy, administer nivolumab as single agent until disease progression or unacceptable toxicity.†
Microsatellite instability-high (MSI‑H) or mismatch repair deficient (dMMR) metastatic colorectal cancer
1 mg/kg every 3 weeks‡
with nivolumab 3 mg/kg‡
After completing 4 doses of combination therapy, administer nivolumab as single agent until disease progression or unacceptable toxicity.†
Hepatocellular carcinoma
3 mg/kg every 3 weeks‡
with nivolumab 1 mg/kg‡
In combination with nivolumab
for 4 doses.After completing 4 doses of combination therapy, administer nivolumab as single agent until disease progression or unacceptable toxicity.†
Metastatic non-small cell lung cancer expressing PD‑L1
1 mg/kg every 6 weeks
with nivolumab 360 mg‡ every 3 weeks
In combination with nivolumab until disease progression, unacceptable toxicity, or up to 2 years in patients without disease progression.†
Metastatic or recurrent non-small cell lung cancer
1 mg/kg every 6 weeks
with nivolumab 360 mg every 3 weeks‡
and histology-based platinum‑doublet
chemotherapy every 3 weeks
In combination with nivolumab until disease progression, unacceptable toxicity, or up to 2 years in patients without disease progression.†
2 cycles of histology-based platinum-doublet chemotherapy
Malignant pleural mesothelioma
1 mg/kg every 6 weeks
with nivolumab 360 mg every 3 weeks‡
In combination with nivolumab until disease progression, unacceptable toxicity, or up to 2 years in patients without disease progression.†
Esophageal squamous cell carcinoma
1 mg/kg every 6 weeks (30-minute intravenous infusion) with nivolumab 3 mg/kg every 2 weeks or 360 mg every 3 weeks (30-minute intravenous infusion)
In combination with nivolumab until disease progression, unacceptable toxicity, or up to 2 years
2.3 Recommended Dosage Modifications for Adverse Reactions
No dose reduction for YERVOY is recommended. In general, withhold YERVOY for severe (Grade 3) immune-mediated adverse reactions. Permanently discontinue YERVOY for life-threatening (Grade 4) immune-mediated adverse reactions, recurrent severe (Grade 3) immune-mediated reactions that require systemic immunosuppressive treatment, persistent moderate (Grade 2) or severe (Grade 3) reactions lasting 12 weeks or longer after last YERVOY dose (excluding endocrinopathy), or an inability to reduce corticosteroid dose to 10 mg or less of prednisone or equivalent per day within 12 weeks of initiating steroids. Dosage modifications for YERVOY or YERVOY in combination with nivolumab for adverse reactions that require management different from these general guidelines are summarized in Table 3.
When YERVOY is administered in combination with nivolumab, withhold or permanently discontinue both YERVOY and nivolumab for toxicity.
Table 3: Recommended Dosage Modifications for Adverse Reactions ALT = alanine aminotransferase, AST = aspartate aminotransferase, DRESS = Drug Rash with Eosinophilia and Systemic Symptoms, SJS = Stevens Johnson Syndrome, TEN = toxic epidermal necrolysis, ULN = upper limit of normal
* Based on Common Terminology Criteria for Adverse Events (CTCAE), Version 4.03
a Resume in patients with complete or partial resolution (Grade 0 or 1) after corticosteroid taper. Permanently discontinue if no complete or partial resolution within 12 weeks of last dose or inability to reduce prednisone to 10 mg per day (or equivalent) or less within 12 weeks of initiating steroids.
b If AST/ALT are less than or equal to ULN at baseline, withhold or permanently discontinue YERVOY based on recommendations for hepatitis with no liver involvement.
c This guidance is only applicable to HCC patients who are being treated with YERVOY in combination with nivolumab.
d Depending on clinical severity, consider withholding for Grade 2 endocrinopathy until symptom improvement with hormone replacement. Resume once acute symptoms have resolved.Adverse Reaction
Severity*
Dosage Modifications
Immune-Mediated Adverse Reactions [See Warnings and Precautions (5.1)]
Colitis
Grade 2
Withholda
Grade 3 or 4
Permanently discontinue
Hepatitis with no tumor involvement of the liver
or
Hepatitis with tumor involvement of the liver/non-HCC
AST or ALT increases to more than 3 times and up to 5 times the ULN
or
Total bilirubin increases to more than 1.5 times and up to 3 times the ULN
Withholda
AST or ALT more than 5 times the ULN
or
Total bilirubin more than 3 times the ULN
Permanently discontinue
Hepatitis with tumor involvement of the liverb/HCCc
Baseline AST/ALT is more than 1 and up to 3 times ULN and increases to more than 5 and up to 10 times ULN
or
Baseline AST/ALT is more than 3 and up to 5 times ULN and increases to more than 8 and up to 10 times ULN.
Withholda
AST/ALT increases to more than 10 times ULN
or
Total bilirubin increases to more than 3 times ULN.
Permanently discontinue
Exfoliative Dermatologic Conditions
Suspected SJS, TEN, or DRESS
Withhold
Confirmed SJS, TEN, or DRESS
Permanently discontinue
Endocrinopathiesd
Grades 3 or 4
Withhold until clinically stable or permanently discontinue depending on severity
Pneumonitis
Grade 2
Withholda
Grade 3 or 4
Permanently discontinue
Nephritis with Renal Dysfunction
Grade 2 or 3 increased blood creatinine
Withholda
Grade 4 increased blood creatinine
Permanently discontinue
Neurological Toxicities
Grade 2
Withholda
Grade 3 or 4
Permanently discontinue
Myocarditis
Grade 2, 3 or 4
Permanently discontinue
Ophthalmologic
Grade 2, 3, or 4 that does not improve to Grade 1 within 2 weeks while receiving topical therapy or that requires systemic treatment
Permanently discontinue
Other Adverse Reactions
Infusion-Related Reactions [see Warnings and Precautions (5.2)]
Grade 1 or 2
Interrupt or slow the rate of infusion
Grade 3 or 4
Permanently discontinue
2.4 Preparation and Administration
- •
- Do not shake product.
- •
- Visually inspect for particulate matter and discoloration prior to administration. Discard vial if solution is cloudy, there is pronounced discoloration (solution may have pale-yellow color), or there is foreign particulate matter other than translucent-to-white, amorphous particles.
Preparation of Solution
- •
- Allow the vial(s) to stand at room temperature for approximately 5 minutes prior to preparation of infusion.
- •
- Withdraw the required volume of YERVOY and transfer into an intravenous bag.
- •
- Dilute with 0.9% Sodium Chloride Injection, USP or 5% Dextrose Injection, USP to a final concentration ranging from 1 mg/mL to 2 mg/mL. Mix diluted solution by gentle inversion.
- •
- After preparation, store the diluted solution either refrigerated at 2°C to 8°C (36°F to 46°F) or at room temperature of 20°C to 25°C (68°F to 77°F) for no more than 24 hours from the time of preparation to the time of infusion.
- •
- Discard partially used or empty vials of YERVOY.
Administration
- •
- Do not co-administer other drugs through the same intravenous line.
- •
- Flush the intravenous line with 0.9% Sodium Chloride Injection, USP or 5% Dextrose Injection, USP after each dose.
- •
- Administer diluted solution through an intravenous line containing a sterile, non-pyrogenic, low-protein-binding in-line filter.
- •
- When administered in combination with nivolumab, infuse nivolumab first followed by YERVOY on the same day. When administered with nivolumab and platinum-doublet chemotherapy, infuse nivolumab first followed by YERVOY and then platinum-doublet chemotherapy on the same day. Use separate infusion bags and filters for each infusion.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Severe and Fatal Immune-Mediated Adverse Reactions
YERVOY is a fully human monoclonal antibody that blocks T-cell inhibitory signals induced by the CTLA-4 pathway, thereby removing inhibition of the immune response with the potential for induction of immune-mediated adverse reactions. Immune-mediated adverse reactions listed herein may not be inclusive of all possible severe and fatal immune-mediated reactions.
Immune-mediated adverse reactions, which may be severe or fatal, can occur in any organ system or tissue. Immune-mediated adverse reactions can occur at any time after starting YERVOY. While immune-mediated adverse reactions usually manifest during treatment, immune-mediated adverse reactions can also manifest after discontinuation of YERVOY.
Early identification and management are essential to ensure safe use of YERVOY. Monitor for signs and symptoms that may be clinical manifestations of underlying immune-mediated adverse reactions. Evaluate clinical chemistries including liver enzymes, creatinine, adrenocorticotropic hormone (ACTH) level, and thyroid function at baseline and before each dose. Institute medical management promptly, including specialty consultation as appropriate.
Withhold or permanently discontinue YERVOY depending on severity [see Dosage and Administration (2.3)]. In general, if YERVOY requires interruption or discontinuation, administer systemic corticosteroid therapy (1 to 2 mg/kg/day prednisone or equivalent) until improvement to Grade 1 or less. Upon improvement to Grade 1 or less, initiate corticosteroid taper and continue to taper over at least 1 month. Consider administration of other systemic immunosuppressants in patients whose immune-mediated adverse reactions are not controlled with corticosteroid therapy.
Immune-Mediated Colitis
YERVOY can cause immune-mediated colitis, which may be fatal. Cytomegalovirus (CMV) infection/reactivation has been reported in patients with corticosteroid-refractory immune-mediated colitis. In cases of corticosteroid-refractory colitis, consider repeating infectious workup to exclude alternative etiologies.
YERVOY 3 mg/kg as a Single Agent
Immune-mediated colitis occurred in 12% (62/511) of patients who received YERVOY 3 mg/kg as a single agent, including Grade 3-5 (7%) and Grade 2 (5%). Colitis led to permanent discontinuation of YERVOY in 4.3% and withholding of at least one dose of YERVOY in 0.2% of patients.
Systemic corticosteroids were required in 74% (46/62) of patients with immune-mediated colitis. Five patients required coadministration of another immunosuppressant with corticosteroids. Colitis resolved in 76% of the 62 patients. One patient was withheld one or more doses of YERVOY for colitis, and no patient received additional treatment after symptom improvement.
YERVOY 10 mg/kg as a Single Agent
Immune-mediated colitis occurred in 31% (144/471) of patients who received YERVOY 10 mg/kg as a single agent, including fatal (0.2%), Grade 4 (1.5%), Grade 3 (14%), and Grade 2 (14%). Colitis led to permanent discontinuation of YERVOY in 61% of patients and 3.8% of patients missed at least one dose of YERVOY due to colitis.
Systemic corticosteroids were required in 85% (123/144) of patients with immune-mediated colitis. Approximately 26% of the 144 patients required coadministration of another immunosuppressant with corticosteroids. Colitis resolved in 90% of the 144 patients. Of the 18 patients who missed one or more doses of YERVOY for colitis, 17 received additional treatment after symptom improvement; of these, 14 had recurrence of colitis.
YERVOY 1 mg/kg with 3 mg/kg Nivolumab
Immune-mediated colitis occurred in 9% (60/666) of patients who received YERVOY 1 mg/kg with nivolumab for the treatment of RCC or mCRC, including Grade 3 (4.4%), and Grade 2 (3.7%). Colitis led to permanent discontinuation of YERVOY and nivolumab in 3.2% and withholding of YERVOY and nivolumab in 2.7% of patients.
In patients who received YERVOY 1 mg/kg with nivolumab, use of systemic corticosteroids was one of the diagnostic criteria required to identify immune-mediated colitis. Systemic corticosteroids were therefore required in 100% (60/60) of patients with immune-mediated colitis. Approximately 23% of patients required coadministration of another immunosuppressant with corticosteroids. Colitis resolved in 95% of the 60 patients. Of the 18 patients in whom YERVOY or nivolumab was withheld for colitis, 16 received additional treatment after symptom improvement; of these, 10 had recurrence of colitis.
YERVOY 3 mg/kg with 1 mg/kg Nivolumab
Immune-mediated colitis occurred in 25% (115/456) of patients with melanoma or HCC receiving YERVOY 3 mg/kg with nivolumab 1 mg/kg every 3 weeks, including Grade 4 (0.4%), Grade 3 (14%), and Grade 2 (8%) adverse reactions. Colitis led to permanent discontinuation of YERVOY with nivolumab in 14% and withholding of treatment in 4.4% of patients.
Systemic corticosteroids were required in 100% (115/115) of patients with colitis. Approximately 23% of patients required addition of infliximab to high-dose corticosteroids. Colitis resolved in 93% of 115 patients. Of the 20 patients in whom YERVOY with nivolumab was withheld for colitis, 16 reinitiated treatment after symptom improvement, and 9 had recurrence of colitis.
Immune-Mediated Hepatitis
YERVOY 3 mg/kg as a Single Agent
Immune-mediated hepatitis occurred in 4.1% (21/511) of patients who received YERVOY 3 mg/kg as a single agent, including Grade 3-5 (1.6%) and Grade 2 (2.5%). Hepatitis led to permanent discontinuation of YERVOY in 0.4% of patients and withholding of at least one dose of YERVOY in none of the patients.
Systemic corticosteroids were required in 29% (6/21) of patients with immune-mediated hepatitis. No patients required the coadministration of another immunosuppressant with corticosteroids. Hepatitis resolved in 86% of the 21 patients.
YERVOY 10 mg/kg as a Single Agent
Immune-mediated hepatitis occurred in 15% (73/471) of patients who received YERVOY 10 mg/kg as a single agent, including Grade 4 (2.8%), Grade 3 (8%), and Grade 2 (5%). Hepatitis led to permanent discontinuation of YERVOY in 56% of patients and 1.1% of patients missed at least one dose of YERVOY due to hepatitis.
Systemic corticosteroids were required in 85% (62/73) of patients with immune-mediated hepatitis. Approximately 15% of the 73 patients required the coadministration of another immunosuppressant with corticosteroids. Hepatitis resolved in 93% of 73 patients. Of the 5 patients who missed one or more doses of YERVOY for hepatitis, 5 received additional treatment after symptom improvement; of these, 1 had recurrence of hepatitis.
YERVOY 3 mg/kg with Vemurafenib
The safety and effectiveness of YERVOY in combination with vemurafenib have not been established [see Indications and Usage (1)]. In a dose-finding trial, Grade 3 increases in transaminases with or without concomitant increases in total bilirubin occurred in 6 of 10 patients who received concurrent YERVOY (3 mg/kg) and vemurafenib (960 mg or 720 mg twice daily).
YERVOY 1 mg/kg with 3 mg/kg Nivolumab
Immune-mediated hepatitis occurred in 7% (48/666) of patients who received YERVOY 1 mg/kg with nivolumab for the treatment of RCC or mCRC, including Grade 4 (1.2%), Grade 3 (4.9%), and Grade 2 (0.4%). Hepatitis led to permanent discontinuation of YERVOY and nivolumab in 3.6% and withholding of YERVOY and nivolumab in 2.6% of patients.
In patients who received YERVOY 1 mg/kg with nivolumab, use of systemic corticosteroids was one of the diagnostic criteria required to identify immune-mediated hepatitis. Systemic corticosteroids were therefore required in 100% (48/48) of patients with immune-mediated hepatitis. Approximately 19% of patients required coadministration of another immunosuppressant with corticosteroids. Hepatitis resolved in 88% of the 48 patients. Of the 17 patients in whom YERVOY or nivolumab was withheld for hepatitis, 14 received additional treatment after symptom improvement; of these, 10 had recurrence of hepatitis.
YERVOY 3 mg/kg with 1 mg/kg Nivolumab
Immune-mediated hepatitis occurred in 15% (70/456) of patients with melanoma or HCC receiving YERVOY 3 mg/kg with nivolumab 1 mg/kg every 3 weeks, including Grade 4 (2.4%), Grade 3 (11%), and Grade 2 (1.8%) adverse reactions. Immune-mediated hepatitis led to permanent discontinuation of YERVOY with nivolumab in 8% and withholding of treatment in 3.5% of patients.
Systemic corticosteroids were required in 100% (70/70) of patients with hepatitis. Approximately 9% of patients with immune-mediated hepatitis required addition of mycophenolic acid to high-dose corticosteroids. Hepatitis resolved in 91% of the 70 patients. Of the 16 patients in whom YERVOY with nivolumab was withheld for hepatitis, 14 reinitiated treatment after symptom improvement, and 8 had recurrence of hepatitis.
Immune-Mediated Dermatologic Adverse Reactions
YERVOY can cause immune-mediated rash or dermatitis, including bullous and exfoliative dermatitis, Stevens Johnson Syndrome, toxic epidermal necrolysis (TEN), and DRESS (Drug Rash with Eosinophilia and Systemic Symptoms). Topical emollients and/or topical corticosteroids may be adequate to treat mild to moderate non-bullous/exfoliative rashes. Withhold or permanently discontinue YERVOY depending on severity [see Dosage and Administration (2.3)].
YERVOY 3 mg/kg as a Single Agent
Immune-mediated rash occurred in 15% (76/511) of patients who received YERVOY 3 mg/kg as a single agent, including Grade 3-5 (2.5%) and Grade 2 (12%). Rash led to permanent discontinuation of YERVOY in 0.2% and withholding of at least one dose of YERVOY in 1.4% of patients.
Systemic corticosteroids were required in 43% (33/76) of patients with immune-mediated rash. Rash resolved in 71% of the 76 patients. Of the 7 patients in whom YERVOY was withheld for rash, 3 received additional treatment after symptom improvement; of these, 1 had recurrence of rash.
YERVOY 10 mg/kg as a Single Agent
Immune-mediated rash occurred in 25% (118/471) of patients who received YERVOY 10 mg/kg as a single agent, including Grade 3 (4%) and Grade 2 (21%). Rash led to permanent discontinuation in 8% of patients and 1.5% of patients missed at least one dose of YERVOY due to rash.
Systemic corticosteroids were required in 70% (83/118) of patients with immune-mediated rash. Rash resolved in 81% of 118 patients. Of the 7 patients who missed one or more doses of YERVOY for rash, 5 received additional treatment after symptom improvement; of these, 3 had recurrence of rash.
YERVOY 1 mg/kg with 3 mg/kg Nivolumab
Immune-mediated rash occurred in 16% (108/666) of patients who received YERVOY 1 mg/kg with nivolumab for the treatment of RCC or mCRC, including Grade 3 (3.5%) and Grade 2 (4.2%). Rash led to permanent discontinuation of YERVOY and nivolumab in 0.5% of patients and withholding of YERVOY and nivolumab in 2.0% of patients.
In patients who received YERVOY 1 mg/kg with nivolumab, use of systemic corticosteroids was one of the diagnostic criteria required to identify immune-mediated rash. Systemic corticosteroids were therefore required in 100% (108/108) of patients. Rash resolved in 75% of 108 patients. Of the 13 patients in whom YERVOY or nivolumab was withheld for rash, 11 received additional treatment after symptom improvement; of these, 5 had recurrence of rash.
YERVOY 3 mg/kg with 1 mg/kg Nivolumab
Immune-mediated rash occurred in 28% (127/456) of patients with melanoma or HCC receiving YERVOY 3 mg/kg with nivolumab 1 mg/kg every 3 weeks, including Grade 3 (4.8%) and Grade 2 (10%) adverse reactions. Immune-mediated rash led to permanent discontinuation of YERVOY with nivolumab in 0.4% and withholding of treatment in 3.9% of patients.
Systemic corticosteroids were required in 100% (127/127) of patients with immune-mediated rash. Rash resolved in 84% of the 127 of patients. Of the 18 patients in whom YERVOY with nivolumab was withheld for rash, 15 reinitiated treatment after symptom improvement, and 8 had recurrence of rash.
Immune-Mediated Endocrinopathies
YERVOY 3 mg/kg as a Single Agent
Grade 2-5 immune-mediated endocrinopathies occurred in 4% (21/511) of patients who received YERVOY 3 mg/kg as a single agent.
Severe to life-threatening (Grade 3-4) endocrinopathies occurred in 9 patients (1.8%). All 9 of these patients had hypopituitarism with some patients having additional concomitant endocrinopathies, such as adrenal insufficiency, hypogonadism, and hypothyroidism. Six of the 9 patients were hospitalized for severe endocrinopathies.
Moderate (Grade 2) endocrinopathy occurred in 12 patients (2.3%), including hypothyroidism, adrenal insufficiency, hypopituitarism, hyperthyroidism and Cushing’s syndrome.
Of the 21 patients with moderate to life-threatening endocrinopathy, 17 required long-term hormone replacement therapy, including adrenal hormones (n=10) and thyroid hormones (n=13).
YERVOY 10 mg/kg as a Single Agent
Immune-mediated endocrinopathies occurred in 28% of patients (132/471), including Grade 4 (0.6%), Grade 3 (8%) and Grade 2 (20%).
Of the 39 patients with Grade 3 to 4 endocrinopathies, 35 patients had hypopituitarism (associated with one or more secondary endocrinopathies, e.g., adrenal insufficiency, hypogonadism, and hypothyroidism), 3 patients had hyperthyroidism, and 1 had primary hypothyroidism. Twenty-seven of the 39 patients (69%) were hospitalized for endocrinopathies. Of the 39 patients, 10% were reported to have resolution.
Of the 93 patients with Grade 2 endocrinopathy, 74 had primary hypopituitarism associated with one or more secondary endocrinopathy, e.g., adrenal insufficiency, hypogonadism, and hypothyroidism, 9 had primary hypothyroidism, 3 had hyperthyroidism, 3 had thyroiditis with hypo- or hyperthyroidism, 2 had hypogonadism, 1 had both hyperthyroidism and hypopituitarism, and 1 subject developed Graves’ ophthalmopathy. Of the 93 patients, 20% were reported to have resolution.
One hundred twenty-four patients received systemic corticosteroids as immunosuppression and/or adrenal hormone replacement for Grade 2 to 4 endocrinopathy. Of these, 42 (34%) were able to discontinue corticosteroids. Seventy-three patients received thyroid hormones for treatment of Grade 2 to 4 hypothyroidism. Of these, 14 patients (19%) were able to discontinue thyroid replacement therapy.
YERVOY 1 mg/kg with 3 mg/kg Nivolumab
Hypophysitis:
YERVOY can cause immune-mediated hypophysitis. Hypophysitis can present with acute symptoms associated with mass effect such as headache, photophobia, or visual field cuts. Hypophysitis can cause hypopituitarism. Initiate hormone replacement as clinically indicated. Withhold or permanently discontinue YERVOY depending on severity [see Dosage and Administration (2.3)].
Hypophysitis occurred in 4.4% (29/666) of patients who received YERVOY 1 mg/kg with nivolumab for the treatment of RCC or mCRC, including Grade 4 (0.3%), Grade 3 (2.4%), and Grade 2 (0.9%). Hypophysitis led to permanent discontinuation of YERVOY and nivolumab in 1.2% and withholding of YERVOY with nivolumab in 2.1% of patients. Approximately 72% of patients with hypophysitis received hormone replacement therapy. Systemic corticosteroids were required in 72% (21/29) of patients with immune-mediated hypophysitis. Hypophysitis resolved in 59% of the 29 patients. Of the 14 patients in whom YERVOY or nivolumab was withheld for hypophysitis, 11 received additional treatment after symptom improvement; of these, 2 had recurrence of hypophysitis.
Adrenal Insufficiency:
Adrenal insufficiency occurred in 7% (48/666) of patients who received YERVOY 1 mg/kg with nivolumab for the treatment of RCC or mCRC, including Grade 4 (0.3%), Grade 3 (2.5%), and Grade 2 (4.1%). Adrenal insufficiency led to permanent discontinuation of YERVOY with nivolumab in 1.2% and withholding of YERVOY with nivolumab in 2.1% of patients. Approximately 94% of patients with adrenal insufficiency received hormone replacement therapy. Systemic corticosteroids were required in 94% (45/48) of patients with adrenal insufficiency. Adrenal insufficiency resolved in 29% of the 48 patients. Of the 14 patients in whom YERVOY or nivolumab was withheld for adrenal insufficiency, 11 received additional treatment after symptom improvement; of these, 2 had recurrence of adrenal insufficiency.
Hyperthyroidism:
Hyperthyroidism occurred in 12% (80/666) of patients who received YERVOY 1 mg/kg with nivolumab for the treatment of RCC or mCRC, including Grade 3 (0.6%) and Grade 2 (4.5%). No patients discontinued YERVOY for hyperthyroidism. Hyperthyroidism led to withholding of YERVOY with nivolumab in 2.3% of patients. Approximately 19% received a thyroid synthesis inhibitor. Systemic corticosteroids were required in 20% (16/80) of patients with hyperthyroidism. Hyperthyroidism resolved in 85% of the 80 patients. Of the 15 patients in whom YERVOY or nivolumab was withheld for hyperthyroidism, 11 received additional treatment after symptom improvement; of these, 3 had recurrence of hyperthyroidism.
Hypothyroidism:
Hypothyroidism occurred in 18% (122/666) of patients who received YERVOY 1 mg/kg with nivolumab for the treatment of RCC or mCRC, including Grade 3 (0.6%) and Grade 2 (11%). Hypothyroidism led to permanent discontinuation of YERVOY with nivolumab in 0.2% and withholding of YERVOY with nivolumab in 1.4% of patients. Approximately 82% received thyroid hormone replacement. Systemic corticosteroids were required in 7% (9/122) of patients with hypothyroidism. Hypothyroidism resolved in 27% of the 122 patients. Of the 9 patients in whom YERVOY or nivolumab was withheld for hypothyroidism, 5 received additional treatment after symptom improvement; of these, one patient had recurrence of hypothyroidism.
Thyroiditis:
Thyroiditis occurred in 2.7% (22/666) of patients who received YERVOY 1 mg/kg with nivolumab for the treatment of RCC or mCRC, including Grade 3 (4.5%) and Grade 2 (2.2%). Thyroiditis led to permanent discontinuation of YERVOY with nivolumab in 0.2% and withholding of YERVOY with nivolumab in 0.8% of patients. Systemic corticosteroids were required in 18% (4/22) of patients with thyroiditis. Thyroiditis resolved in 64% of the 22 patients. Of the 5 patients in whom YERVOY or nivolumab was withheld for thyroiditis, 5 received additional treatment after symptom improvement; of these, no patients had recurrence of thyroiditis.
Type 1 Diabetes Mellitus:
Diabetes occurred in 2.7% (15/666) of patients who received YERVOY 1 mg/kg with nivolumab for the treatment of RCC or mCRC, including Grade 4 (0.6%), Grade 3 (0.3%), and Grade 2 (0.9%). Diabetes led to the permanent discontinuation of YERVOY with nivolumab in 0.5% and withholding of YERVOY with nivolumab in 0.5% of patients. Systemic corticosteroids were required in 7% (1/15) of patients with diabetes. Diabetes resolved in 27% of the 15 patients. Of the 3 patients in whom YERVOY or nivolumab was withheld for diabetes, 2 received additional treatment after symptom improvement; of these, none had recurrence of diabetes.
YERVOY 3 mg/kg with 1 mg/kg Nivolumab
Hypophysitis:
Hypophysitis occurred in 9% (42/456) of patients with melanoma or HCC receiving YERVOY 3 mg/kg with nivolumab 1 mg/kg every 3 weeks, including Grade 3 (2.4%) and Grade 2 (6%) adverse reactions. Hypophysitis led to permanent discontinuation of YERVOY with nivolumab in 0.9% and withholding of treatment in 4.2% of patients.
Approximately 86% of patients with hypophysitis received hormone replacement therapy. Systemic corticosteroids were required in 88% (37/42) of patients with hypophysitis. Hypophysitis resolved in 38% of the 42 patients. Of the 19 patients in whom YERVOY with nivolumab was withheld for hypophysitis, 9 reinitiated treatment after symptom improvement, and 1 had recurrence of hypophysitis.
Adrenal Insufficiency:
Adrenal insufficiency occurred in 8% (35/456) of patients with melanoma or HCC receiving YERVOY 3 mg/kg with nivolumab 1 mg/kg every 3 weeks, including Grade 4 (0.2%), Grade 3 (2.4%), and Grade 2 (4.2%) adverse reactions. Adrenal insufficiency led to permanent discontinuation of YERVOY with nivolumab in 0.4% of patients and withholding of treatment in 2.0% of patients.
Approximately 71% (25/35) of patients with adrenal insufficiency received hormone replacement therapy, including systemic corticosteroids. Adrenal insufficiency resolved in 37% of the 35 patients. Of the 9 patients in whom YERVOY with nivolumab was withheld for adrenal insufficiency, 7 reinitiated treatment after symptom improvement, and all required hormone replacement therapy for their ongoing adrenal insufficiency.
Hypothyroidism:
Hypothyroidism occurred in 20% (91/456) of patients with melanoma or HCC receiving YERVOY 3 mg/kg with nivolumab 1 mg/kg every 3 weeks, including Grade 3 (0.4%) and Grade 2 (11%) adverse reactions. Hypothyroidism led to permanent discontinuation of YERVOY with nivolumab in 0.9% of patients and withholding of treatment in 0.9% of patients.
Approximately 89% of patients with hypothyroidism received levothyroxine. Systemic corticosteroids were required in 2.2% (2/91) of patients with hypothyroidism. Hypothyroidism resolved in 41% of the 91 patients. Of the 4 patients in whom YERVOY with nivolumab was withheld for hypothyroidism, 2 reinitiated treatment after symptom improvement, and none had recurrence of hypothyroidism.
Hyperthyroidism:
Hyperthyroidism occurred in 9% (42/456) of patients with melanoma or HCC receiving YERVOY 3 mg/kg with nivolumab 1 mg/kg every 3 weeks, including Grade 3 (0.9%) and Grade 2 (4.2%) adverse reactions. Hyperthyroidism led to permanent discontinuation of YERVOY with nivolumab in no patients and withholding of treatment in 2.4% of patients.
Approximately 26% of patients with hyperthyroidism received methimazole and 21% received carbimazole. Systemic corticosteroids were required in 17% (7/42) of patients. Hyperthyroidism resolved in 91% of the 42 patients. Of the 11 patients in whom YERVOY with nivolumab was withheld for hyperthyroidism, 8 reinitiated treatment after symptom improvement, and 1 had recurrence of hyperthyroidism.
Immune-Mediated Pneumonitis
YERVOY 1 mg/kg with 3 mg/kg Nivolumab
Immune-mediated pneumonitis occurred in 3.9% (26/666) of patients who received YERVOY 1 mg/kg with nivolumab for the treatment of RCC or mCRC, including Grade 3 (1.4%) and Grade 2 (2.6%). Pneumonitis led to permanent discontinuation of YERVOY and nivolumab in 1.8% and withholding of YERVOY and nivolumab in 1.5% of patients.
In patients who received YERVOY 1 mg/kg with nivolumab, use of systemic corticosteroids was one of the diagnostic criteria required to identify immune-mediated pneumonitis. Systemic corticosteroids were therefore required in 100% (26/26) of patients with immune-mediated pneumonitis. Approximately 8% required coadministration of another immunosuppressant with corticosteroids. Pneumonitis resolved in 92% of the 26 patients. Of the 10 patients in whom YERVOY or nivolumab was withheld for pneumonitis, 10 received additional treatment after symptom improvement; of these, 4 had recurrence of pneumonitis.
In NSCLC, immune-mediated pneumonitis occurred in 9% (50/576) of patients receiving YERVOY 1 mg/kg every 6 weeks with nivolumab 3 mg/kg every 2 weeks, including Grade 4 (0.5%), Grade 3 (3.5%), and Grade 2 (4.0%) immune-mediated pneumonitis. Four patients (0.7%) died due to pneumonitis. The median duration was 1.5 months (range: 5 days to 25+ months). Immune-mediated pneumonitis led to permanent discontinuation of YERVOY with nivolumab in 5% of patients and withholding of YERVOY with nivolumab in 3.6% of patients.
Systemic corticosteroids were required in 100% of patients with pneumonitis followed by a corticosteroid taper. Pneumonitis resolved in 72% of the patients. Approximately 13% (2/16) of patients had recurrence of pneumonitis after re-initiation of YERVOY with nivolumab.
YERVOY 3 mg/kg with 1 mg/kg Nivolumab
Immune-mediated pneumonitis occurred in 7% (31/456) of patients who received YERVOY 3 mg/kg with nivolumab for the treatment of HCC or melanoma, including Grade 4 (0.2%), Grade 3 (2.0%), and Grade 2 (4.4%). Immune-mediated pneumonitis led to permanent discontinuation or withholding of treatment in 2.9% and 3.9% of patients, respectively.
Systemic corticosteroids were required in 100% of patients with pneumonitis. Pneumonitis resolved in 94% of the patients. Of the 13 patients in whom YERVOY or nivolumab was withheld for pneumonitis, 13 received additional treatment after symptom improvement, and 4 had recurrence of pneumonitis.
Immune-Mediated Nephritis with Renal Dysfunction
YERVOY 1 mg/kg with 3 mg/kg Nivolumab
Immune-mediated nephritis with renal dysfunction occurred in 4.1% (27/666) of patients who received YERVOY 1 mg/kg with nivolumab for the treatment of RCC or mCRC, including Grade 4 (0.6%), Grade 3 (1.1%), and Grade 2 (2.2%). Nephritis with renal dysfunction led to permanent discontinuation of YERVOY and nivolumab in 1.2% and withholding of nivolumab and YERVOY in 1.8% of patients.
In patients who received YERVOY 1 mg/kg with nivolumab, use of systemic corticosteroids was one of the diagnostic criteria required to identify immune-mediated nephritis with renal dysfunction. Systemic corticosteroids were therefore required in 100% (27/27) of patients with immune-mediated nephritis with renal dysfunction. Nephritis with renal dysfunction resolved in 67% of the 27 patients. Of the 12 patients in whom YERVOY or nivolumab was withheld for nephritis, 10 received additional treatment after symptom improvement; of these, 4 had recurrence of nephritis.
Other Immune-Mediated Adverse Reactions
Across clinical trials of YERVOY administered as a single agent or in combination with nivolumab, the following clinically significant immune-mediated adverse reactions, some with fatal outcome, occurred in <1% of patients unless otherwise specified, as shown below:
Nervous System: Autoimmune neuropathy (2%), meningitis, encephalitis, myelitis and demyelination, myasthenic syndrome/myasthenia gravis, Guillain-Barré syndrome, nerve paresis, motor dysfunction
Cardiovascular: Angiopathy, myocarditis, pericarditis, temporal arteritis, vasculitis
Ocular: Blepharitis, episcleritis, iritis, orbital myositis, scleritis, uveitis. Some cases can be associated with retinal detachment. If uveitis occurs in combination with other immune-mediated adverse reactions, consider a Vogt-Koyanagi-Harada-like syndrome, which has been observed in patients receiving YERVOY and may require treatment with systemic corticosteroids to reduce the risk of permanent vision loss.
Gastrointestinal: Duodenitis, gastritis, pancreatitis (1.3%)
Musculoskeletal and Connective Tissue: Arthritis, myositis, polymyalgia rheumatica, polymyositis, rhabdomyolysis
Other (hematologic/immune): Aplastic anemia, conjunctivitis, cytopenias (2.5%), eosinophilia (2.1%), erythema multiforme, histiocytic necrotizing lymphadenitis (Kikuchi lymphadenitis), hypersensitivity vasculitis, meningitis, neurosensory hypoacusis, psoriasis, sarcoidosis, systemic inflammatory response syndrome, and solid organ transplant rejection.
5.2 Infusion-Related Reactions
Severe infusion-related reactions can occur with YERVOY. Discontinue YERVOY in patients with severe or life-threatening infusion reactions. Interrupt or slow the rate of infusion in patients with mild or moderate infusion reactions [see Dosage and Administration (2.3)]. Infusion-related reactions occurred in 2.9% (28/982) of patients who received single-agent YERVOY 3 mg/kg or 10 mg/kg for the treatment of melanoma. Infusion-related reactions occurred in 5% (33/666) of patients who received YERVOY 1 mg/kg with nivolumab for the treatment of RCC or CRC. Infusion-related reactions occurred in 8% (4/49) of patients who received YERVOY 3 mg/kg with nivolumab for the treatment of HCC. Infusion-related reactions occurred in 12% (37/300) of patients with malignant pleural mesothelioma who received YERVOY 1 mg/kg every 6 weeks with nivolumab 3 mg/kg every 2 weeks.
5.3 Complications of Allogeneic Hematopoietic Stem Cell Transplant after YERVOY
Fatal or serious graft-versus-host disease (GVHD) can occur in patients who receive YERVOY either before or after allogeneic hematopoietic stem cell transplantation (HSCT). These complications may occur despite intervening therapy between CTLA-4 receptor blocking antibody and allogeneic HSCT.
Follow patients closely for evidence of GVHD and intervene promptly [see Adverse Reactions (6.3)]. Consider the benefit versus risks of treatment with YERVOY after allogeneic HSCT.
5.4 Embryo-Fetal Toxicity
Based on its mechanism of action and findings from animal studies, YERVOY can cause fetal harm when administered to a pregnant woman. In animal reproduction studies, administration of ipilimumab to cynomolgus monkeys from the onset of organogenesis through delivery resulted in higher incidences of abortion, stillbirth, premature delivery (with corresponding lower birth weight) and higher incidences of infant mortality in a dose-related manner. The effects of ipilimumab are likely to be greater during the second and third trimesters of pregnancy. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with YERVOY and for 3 months after the last dose [see Use in Specific Populations (8.1, 8.3)].
5.5 Risks Associated When Administered in Combination with Nivolumab
YERVOY is indicated for use in combination with nivolumab for patients with advanced RCC, MSI-H or dMMR mCRC, HCC, and NSCLC. Refer to the nivolumab Full Prescribing Information for additional risk information that applies to the combination use treatment.
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- •
- Severe and fatal immune-mediated adverse reactions [see Warnings and Precautions (5.1)].
- •
- Infusion-related reactions [see Warnings and Precautions (5.2)].
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The data described in the Warnings and Precautions section reflect exposure to YERVOY 3 mg/kg as a single agent (or in combination with an investigational gp100 peptide vaccine) in 511 patients in Study MDX010-20; YERVOY 10 mg/kg as a single agent in 471 patients in Study CA184-029; YERVOY 1 mg/kg administered with nivolumab 3 mg/kg in 1,362 patients in CHECKMATE-214, CHECKMATE-142, CHECKMATE-227, and CHECKMATE-743; YERVOY 3 mg/kg administered with nivolumab 1 mg/kg in 456 patients enrolled in CHECKMATE-067, CHECKMATE-040, and another randomized trial; and to YERVOY 1 mg/kg, administered in combination with nivolumab and platinum-doublet chemotherapy in CHECKMATE-9LA.
Unresectable or Metastatic Melanoma
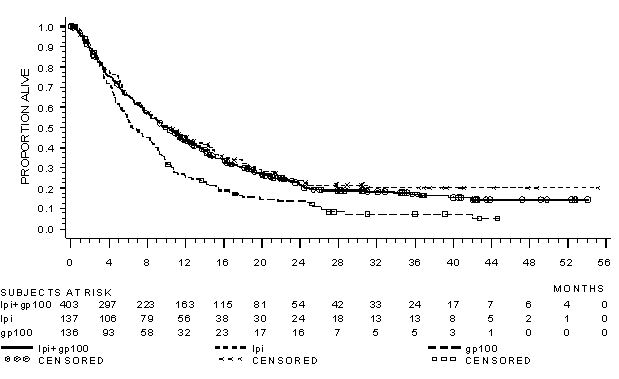
The safety of YERVOY was evaluated in 643 previously treated patients with unresectable or metastatic melanoma in Study MDX010-20 [see Clinical Studies (14.1)]. Study MDX010-20 excluded patients with active autoimmune disease or those receiving systemic immunosuppression for organ transplantation. Patients received YERVOY 3 mg/kg by intravenous infusion for 4 doses as a single agent (n=131), YERVOY with an investigational gp100 peptide vaccine (n=380), or gp100 peptide vaccine as a single agent (n=132). Patients in the trial received a median of 4 doses (range: 1 to 4 doses).
The trial population characteristics were: median age 57 years (range: 19 to 90), 59% male, 94% White, and baseline ECOG performance status 0 (56%).
YERVOY was discontinued for adverse reactions in 10% of patients. Table 4 presents adverse reactions from Study MDX010-20.
Table 4: Selected Adverse Reactions (≥ 5%) in Patients Receiving YERVOY with a Difference Between Arms of >5% for All Grades and >1% for Grades 3 to 5 Compared to gp100 Peptide Vaccine in Study MDX010-20 Adverse Reactions
YERVOY 3 mg/kg
n=131
YERVOY 3 mg/kg and gp100
n=380
gp100
n=132
All Grades (%)
Grade
3 to 5 (%)All Grades
(%)
Grade
3 to 5 (%)All Grades (%)
Grade
3 to 5 (%)General and Administration-Site Conditions
Fatigue
41
7
34
5
31
3
Gastrointestinal
Diarrhea
32
5
37
4
20
1
Colitis
8
5
5
3
2
0
Dermatologic
Pruritus
31
0
21
<1
11
0
Rash
29
2
25
2
8
0
Unresectable or Metastatic Melanoma: In Combination with Nivolumab
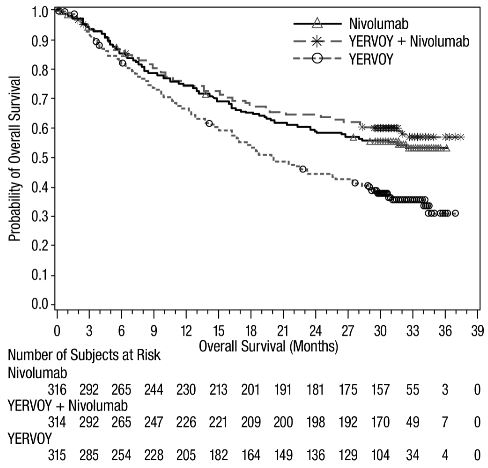
The safety of YERVOY, administered with nivolumab or as a single agent, was evaluated in CHECKMATE-067, a randomized (1:1:1), double-blind trial in 937 patients with previously untreated, unresectable or metastatic melanoma [see Clinical Studies (14.1)]. The trial excluded patients with autoimmune disease, a medical condition requiring systemic treatment with corticosteroids (more than 10 mg daily prednisone equivalent) or other immunosuppressive medication within 14 days of the start of study therapy, a positive test result for hepatitis B or C, or a history of HIV.
Patients were randomized to receive:
- •
- YERVOY 3 mg/kg by intravenous infusion over 90 minutes with nivolumab 1 mg/kg by intravenous infusion every 3 weeks for 4 doses followed by nivolumab as a single agent at a dose of 3 mg/kg by intravenous infusion every 2 weeks (YERVOY and nivolumab arm; n=313), or
- •
- Nivolumab 3 mg/kg by intravenous infusion every 2 weeks (nivolumab arm; n=313), or
- •
- YERVOY 3 mg/kg by intravenous infusion over 90 minutes every 3 weeks for up to 4 doses (YERVOY arm; n=311).
The median duration of exposure to nivolumab was 2.8 months (range: 1 day to 36.4 months) for the YERVOY and nivolumab arm. In the YERVOY and nivolumab arm, 39% were exposed to nivolumab for ≥6 months and 30% exposed for >1 year.
Serious adverse reactions (74%), adverse reactions leading to permanent discontinuation (47%) or to dosing delays (58%), and Grade 3 or 4 adverse reactions (72%) occurred in patients treated with YERVOY and nivolumab.
The most frequent (≥10%) serious adverse reactions in the YERVOY and nivolumab arm were diarrhea (13%), colitis (10%), and pyrexia (10%). The most frequent adverse reactions leading to discontinuation of both drugs in the YERVOY and nivolumab arm were colitis (10%), diarrhea (8%), increased ALT (4.8%), increased AST (4.5%), and pneumonitis (1.9%).
The most common (≥20%) adverse reactions in the YERVOY and nivolumab arm were fatigue, diarrhea, rash, nausea, pyrexia, pruritus, musculoskeletal pain, vomiting, decreased appetite, cough, headache, dyspnea, upper respiratory tract infection, arthralgia, and increased transaminases.
Tables 5 and 6 summarize the incidence of adverse reactions and laboratory abnormalities, respectively, in CHECKMATE-067.
Table 5: Adverse Reactions Occurring in ≥10% of Patients on the YERVOY and Nivolumab Arm or the Nivolumab Arm and at a Higher Incidence than in the YERVOY Arm (Between Arm Difference of ≥5% All Grades or ≥2% Grades 3-4) - CHECKMATE-067 Toxicity was graded per NCI CTCAE v4.
a Includes asthenia and fatigue.
b Includes pustular rash, dermatitis, acneiform dermatitis, allergic dermatitis, atopic dermatitis, bullous dermatitis, exfoliative dermatitis, psoriasiform dermatitis, drug eruption, exfoliative rash, erythematous rash, generalized rash, macular rash, maculopapular rash, morbilliform rash, papular rash, papulosquamous rash, and pruritic rash.
c Includes back pain, bone pain, musculoskeletal chest pain, musculoskeletal discomfort, myalgia, neck pain, pain in extremity, and spinal pain.
d Includes upper respiratory tract infection, nasopharyngitis, pharyngitis, and rhinitis.
e Includes hypertension and blood pressure increased.Adverse Reaction
YERVOY and
Nivolumab
(n=313)
Nivolumab(n=313)
YERVOY(n=311)
All Grades (%)
Grades
3-4 (%)All Grades (%)
Grades
3-4 (%)All Grades (%)
Grades
3-4 (%)General
Fatiguea
62
7
59
1.6
51
4.2
Pyrexia
40
1.6
16
0
18
0.6
Gastrointestinal
Diarrhea
54
11
36
5
47
7
Nausea
44
3.8
30
0.6
31
1.9
Vomiting
31
3.8
20
1.0
17
1.6
Skin and Subcutaneous Tissue
Rashb
53
6
40
1.9
42
3.5
Vitiligo
9
0
10
0.3
5
0
Musculoskeletal and Connective Tissue
Musculoskeletal painc
32
2.6
42
3.8
36
1.9
Arthralgia
21
0.3
21
1.0
16
0.3
Metabolism and Nutrition
Decreased appetite
29
1.9
22
0
24
1.3
Respiratory, Thoracic and Mediastinal
Cough/productive cough
27
0.3
28
0.6
22
0
Dyspnea/exertional dyspnea
24
2.9
18
1.3
17
0.6
Infections
Upper respiratory tract infectiond
23
0
22
0.3
17
0
Endocrine
Hypothyroidism
19
0.6
11
0
5
0
Hyperthyroidism
11
1.3
6
0
1
0
Investigations
Decreased weight
12
0
7
0
7
0.3
Vascular
Hypertensione
7
2.2
11
5
9
2.3
Clinically important adverse reactions in <10% of patients who received YERVOY with nivolumab:
Gastrointestinal Disorders: stomatitis, intestinal perforation
Skin and Subcutaneous Tissue Disorders: vitiligo
Musculoskeletal and Connective Tissue Disorders: myopathy, Sjogren’s syndrome, spondyloarthropathy, myositis (including polymyositis)
Nervous System Disorders: neuritis, peroneal nerve palsy
Table 6: Laboratory Abnormalities Worsening from Baselinea Occurring in ≥20% of Patients Treated with YERVOY with Nivolumab or Single-Agent Nivolumab and at a Higher Incidence than in the YERVOY Arm (Between Arm Difference of ≥5% All Grades or ≥2% Grades 3-4) - CHECKMATE-067 a Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: YERVOY and nivolumab (range: 75 to 297); nivolumab (range: 81 to 306); YERVOY (range: 61 to 301) Laboratory Abnormality
YERVOY and
Nivolumab
Nivolumab
YERVOYAll Grades (%)
Grade
3-4 (%)All Grades (%)
Grade
3-4 (%)All Grades (%)
Grade
3-4 (%)Chemistry
Increased ALT
55
16
25
3.0
29
2.7
Hyperglycemia
53
5
46
7
26
0
Increased AST
52
13
29
3.7
29
1.7
Hyponatremia
45
10
22
3.3
26
7
Increased lipase
43
22
32
12
24
7
Increased alkaline phosphatase
41
6
27
2.0
23
2.0
Hypocalcemia
31
1.1
15
0.7
20
0.7
Increased amylase
27
10
19
2.7
15
1.6
Increased creatinine
26
2.7
19
0.7
17
1.3
Hematology
Anemia
52
2.7
41
2.6
41
6
Lymphopenia
39
5
41
4.9
29
4.0
Adjuvant Treatment of Melanoma
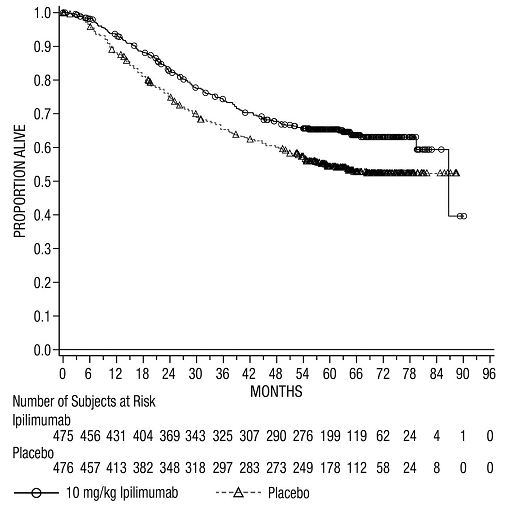
The safety of YERVOY was evaluated in 945 patients with resected Stage IIIA (>1 mm nodal involvement), IIIB, and IIIC (with no in-transit metastases) cutaneous melanoma in Study CA184-029 [see Clinical Studies (14.2)]. Study CA184-029 excluded patients with prior systemic therapy for melanoma, autoimmune disease, a condition requiring systemic immunosuppression, or a positive test for hepatitis B, hepatitis C, or HIV. Patients received YERVOY 10 mg/kg (n=471) or placebo (n=474) administered as an intravenous infusion for 4 doses every 3 weeks followed by 10 mg/kg every 12 weeks beginning at Week 24 up to a maximum of 3 years. In this trial, 36% of patients received YERVOY for longer than 6 months and 26% of patients received YERVOY for longer than 1 year. YERVOY-treated patients in the trial received a median of 4 doses (range: 1 to 16).
The trial population characteristics were: median age 51 years (range: 18 to 84 years), 62% male, 99% White, and baseline ECOG performance status 0 (94%).
YERVOY was discontinued for adverse reactions in 52% of patients. Table 7 presents selected adverse reactions from Study CA184-029.
Table 7: Adverse Reactions (≥ 5%) in Patients Receiving YERVOY with a Difference Between Arms >5% Compared to Placebo in Study CA184-029 Adverse Reaction
YERVOY 10 mg/kg
n=471Placebo
n=474All Grades (%)
Grade 3 to 5 (%)
All Grades (%)
Grade 3 to 5 (%)
Dermatologic
Rash
50
2.1
20
0
Pruritus
45
2.3
15
0
Gastrointestinal
Diarrhea
49
10
30
2.1
Nausea
25
0.2
18
0
Colitis
16
8
1.5
0.4
Vomiting
13
0.4
6
0.2
General and Administration-Site Conditions
Fatigue
46
2.3
38
1.5
Weight Decreased
32
0.2
9
0.4
Pyrexia
18
1.1
4.9
0.2
Nervous System
Headache
33
0.8
18
0.2
Metabolism and Nutrition
Decreased Appetite
14
0.2
3.4
0.2
Psychiatric
Insomnia
10
0
4.4
0
Table 8 presents selected laboratory abnormalities from Study CA184-029.
Table 8: Laboratory Abnormalities (>5%) Worsening from Baseline in Patients Receiving YERVOY with a Difference Between Arms of >5% Compared to Placebo in CA184-029a a Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available. Excluding lipase and amylase, YERVOY group (range: 466 to 470 patients) and placebo group (range: 472 to 474 patients). For lipase and amylase, YERVOY group (range: 447 to 448 patients) and placebo group (range: 462 to 464 patients). Laboratory Abnormality
YERVOY 10 mg/kga
Placeboa
All Grades
(%)Grade 3 to 4
(%)
All Grades
(%)Grade 3 to 4
(%)Chemistry
Increased ALT
46
10
16
0
Increased AST
38
9
14
0.2
Increased lipase
26
9
17
4.5
Increased amylase
17
2.0
7
0.6
Increased alkaline phosphatase
17
0.6
6
0.2
Increased bilirubin
11
1.5
9
0
Increased creatinine
10
0.2
6
0
Hematology
Decreased hemoglobin
25
0.2
14
0
Other Clinical Experience
Across clinical studies in which patients received YERVOY as a single agent at doses ranging from 0.3 to 10 mg/kg, the following adverse reactions were also reported (incidence <1% unless otherwise noted): urticaria (2%), large intestinal ulcer, esophagitis, acute respiratory distress syndrome, renal failure, and infusion reaction.
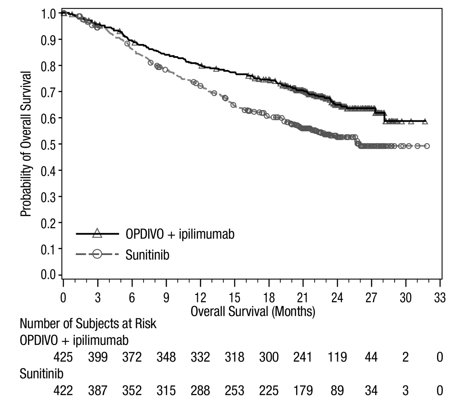
Advanced Renal Cell Carcinoma: In Combination with Nivolumab
The safety of YERVOY in combination with nivolumab was evaluated in 1082 patients with previously untreated advanced RCC in CHECKMATE-214 [see Clinical Studies (14.3)]. Patients received YERVOY 1 mg/kg with nivolumab 3 mg/kg intravenously every 3 weeks for 4 doses followed by nivolumab as a single agent at a dose of 3 mg/kg every 2 weeks (n=547) or sunitinib 50 mg orally daily for first 4 weeks of each 6-week cycle (n=535). The median duration of treatment was 7.9 months (range: 1 day to 21.4+ months) in YERVOY and nivolumab arm. In this trial, 57% of patients in the YERVOY and nivolumab arm were exposed to treatment for greater than 6 months and 38% of patients were exposed to treatment for greater than 1 year.
Serious adverse reactions occurred in 59% of patients receiving YERVOY with nivolumab. The most frequent serious adverse reactions reported in ≥2% of patients treated with YERVOY and nivolumab were diarrhea, pyrexia, pneumonia, pneumonitis, hypophysitis, acute kidney injury, dyspnea, adrenal insufficiency, and colitis.
In patients who received YERVOY with nivolumab, study therapy was discontinued for adverse reactions in 31% and delayed for adverse reactions in 54%.
The most common adverse reactions (≥20%) in the YERVOY and nivolumab arm were fatigue, rash, diarrhea, musculoskeletal pain, pruritus, nausea, cough, pyrexia, arthralgia, vomiting, dyspnea, and decreased appetite. Table 9 summarizes adverse reactions in CHECKMATE-214.
Table 9: Adverse Reactions (>15%) in Patients Receiving YERVOY and Nivolumab in CHECKMATE-214 Toxicity was graded per NCI CTCAE v4.
a Includes asthenia.
b Includes peripheral edema, peripheral swelling.
c Includes dermatitis described as acneiform, bullous, and exfoliative, drug eruption, rash described as exfoliative, erythematous, follicular, generalized, macular, maculopapular, papular, pruritic, and pustular, fixed-drug eruption.
d Includes back pain, bone pain, musculoskeletal chest pain, musculoskeletal discomfort, myalgia, neck pain, pain in extremity, spinal pain.Adverse Reaction
YERVOY 1 mg/kg and Nivolumab
n=547Sunitinib
n=535Grades 1-4
(%)
Grades 3-4
(%)
Grades 1-4
(%)
Grades 3-4
(%)
General and Administration Site Conditions
Fatiguea
58
8
69
13
Pyrexia
25
0.7
17
0.6
Edemab
16
0.5
17
0.6
Skin and Subcutaneous Tissue
Rashc
39
3.7
25
1.1
Pruritus/generalized pruritus
33
0.5
11
0
Gastrointestinal
Diarrhea
38
4.6
58
6
Nausea
30
2.0
43
1.5
Vomiting
20
0.9
28
2.1
Abdominal pain
19
1.6
24
1.9
Constipation
17
0.4
18
0
Musculoskeletal and Connective Tissue
Musculoskeletal paind
37
4.0
40
2.6
Arthralgia
23
1.3
16
0
Respiratory, Thoracic, and Mediastinal
Cough/productive cough
28
0.2
25
0.4
Dyspnea/exertional dyspnea
20
2.4
21
2.1
Metabolism and Nutrition
Decreased appetite
21
1.8
29
0.9
Nervous System
Headache
19
0.9
23
0.9
Endocrine
Hypothyroidism
18
0.4
27
0.2
Table 10 summarizes the laboratory abnormalities in CHECKMATE-214.
Table 10: Laboratory Abnormalities (>15%) Worsening from Baseline in Patients Receiving YERVOY and Nivolumab in CHECKMATE-214 a Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: nivolumab and YERVOY group (range: 490 to 538 patients) and sunitinib group (range: 485 to 523 patients). Laboratory Abnormality
YERVOY 1 mg/kg and Nivolumaba
Sunitiniba
Grades 1-4
(%)Grades 3-4
(%)Grades 1-4
(%)Grades 3-4
(%)Chemistry
Increased lipase
48
20
51
20
Increased creatinine
42
2.1
46
1.7
Increased ALT
41
7
44
2.7
Increased AST
40
4.8
60
2.1
Increased amylase
39
12
33
7
Hyponatremia
39
10
36
7
Increased alkaline phosphatase
29
2.0
32
1.0
Hyperkalemia
29
2.4
28
2.9
Hypocalcemia
21
0.4
35
0.6
Hypomagnesemia
16
0.4
26
1.6
Hematology
Anemia
43
3.0
64
9
Lymphopenia
36
5
63
14
In addition, among patients with TSH ≤ ULN at baseline, a lower proportion of patients experienced a treatment-emergent elevation of TSH > ULN in the YERVOY with nivolumab group compared to the sunitinib group (31% and 61%, respectively).
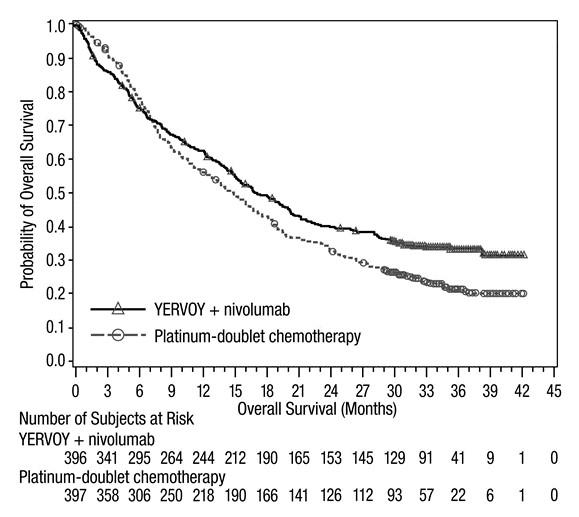
MSI-H or dMMR Metastatic Colorectal Cancer: In Combination with Nivolumab
The safety of YERVOY with nivolumab was evaluated in 119 patients with previously treated MSI-H or dMMR mCRC in a single-arm cohort of CHECKMATE-142 [see Clinical Studies (14.4)]. All patients had received prior fluorouracil-based chemotherapy for metastatic disease; 69% had received prior treatment with a fluoropyrimidine, oxaliplatin, and irinotecan and 29% had received an anti-EGFR antibody. Patients received YERVOY 1 mg/kg and nivolumab 3 mg/kg on Day 1 of each 21-day cycle for 4 doses, then nivolumab 3 mg/kg every 2 weeks until disease progression or unacceptable toxicity. The median duration of exposure for YERVOY was 2.1 months.
Serious adverse reactions occurred in 47% of patients receiving YERVOY and nivolumab. The most frequent serious adverse reactions reported in ≥2% of patients were colitis/diarrhea, hepatic events, abdominal pain, acute kidney injury, pyrexia, and dehydration.
The most common adverse reactions (≥20%) in the YERVOY and nivolumab cohort were fatigue, diarrhea, pyrexia, musculoskeletal pain, abdominal pain, pruritus, nausea, rash, decreased appetite, and vomiting. Table 11 summarizes adverse reactions in CHECKMATE-142.
Table 11: Adverse Reactions Occurring in ≥10% of Patients (CHECKMATE-142) Toxicity was graded per NCI CTCAE v4.
a Includes asthenia.
b Includes peripheral edema and peripheral swelling.
c Includes upper abdominal pain, lower abdominal pain, and abdominal discomfort.
d Includes back pain, pain in extremity, myalgia, neck pain, and bone pain.
e Includes dermatitis, dermatitis acneiform, and rash described as maculo-papular, erythematous, and generalized.
f Includes nasopharyngitis and rhinitis.Adverse Reaction
YERVOY and Nivolumab MSI-H/dMMR Cohort
(n=119)
All Grades (%)
Grades 3-4 (%)
General and Administration Site Conditions
Fatiguea
49
6
Pyrexia
36
0
Edemab
7
0
Gastrointestinal
Diarrhea
45
3.4
Abdominal painc
30
5
Nausea
26
0.8
Vomiting
20
1.7
Constipation
15
0
Musculoskeletal and Connective Tissue
Musculoskeletal paind
36
3.4
Arthralgia
14
0.8
Skin and Subcutaneous Tissue
Pruritus
28
1.7
Rashe
25
4.2
Dry Skin
11
0
Infections and Infestations
Upper respiratory tract infectionf
9
0
Metabolism and Nutrition
Decreased appetite
20
1.7
Respiratory, Thoracic, and Mediastinal
Cough
19
0.8
Dyspnea
13
1.7
Nervous System
Headache
17
1.7
Dizziness
11
0
Endocrine
Hyperglycemia
6
1
Hypothyroidism
14
0.8
Hyperthyroidism
12
0
Investigations
Weight decreased
10
0
Psychiatric
Insomnia
13
0.8
Other clinically important adverse reactions reported in <10% of patients receiving YERVOY in CHECKMATE-142 were encephalitis (0.8%), necrotizing myositis (0.8%), and uveitis (0.8%).
Table 12 summarizes laboratory abnormalities in CHECKMATE-142.
Table 12: Laboratory Abnormalities Worsening from Baselinea Occurring in ≥10% of Patients (CHECKMATE-142) a Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available. Number of evaluable patients ranges from 87 to 114 for nivolumab with YERVOY and from 62 to 71 for nivolumab. Laboratory Abnormality
YERVOY and Nivolumab MSI-H/dMMR Cohort
(n=119)All Grades (%)
Grades 3-4 (%)
Hematology
Anemia
42
9
Thrombocytopenia
26
0.9
Lymphopenia
25
6
Neutropenia
18
0
Chemistry
Increased AST
40
12
Increased lipase
39
12
Increased amylase
36
3.4
Increased ALT
33
12
Increased alkaline phosphatase
28
5
Hyponatremia
26
5
Increased creatinine
25
3.6
Hyperkalemia
23
0.9
Increased bilirubin
21
5
Hypomagnesemia
18
0
Hypocalcemia
16
0
Hypokalemia
15
1.8
Hepatocellular Carcinoma: In Combination with Nivolumab
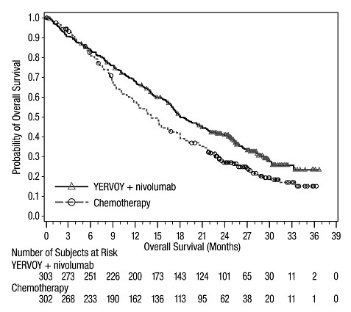
The safety of YERVOY 3 mg/kg in combination with nivolumab 1 mg/kg was evaluated in a subgroup of 49 patients with HCC and Child-Pugh Class A cirrhosis who progressed on or were intolerant to sorafenib enrolled in Cohort 4 of CHECKMATE-040. YERVOY and nivolumab were administered every 3 weeks for four doses, followed by single-agent nivolumab 240 mg every 2 weeks until disease progression or unacceptable toxicity.
During the YERVOY and nivolumab combination period, 33 of 49 (67%) patients received all four planned doses of YERVOY and nivolumab. During the entire treatment period, the median duration of exposure to YERVOY was 2.1 months (range: 0 to 4.5 months) and to nivolumab was 5.1 months (range: 0 to 35+ months). Forty-seven percent of patients were exposed to treatment for >6 months, and 35% of patients were exposed to treatment for >1 year. Serious adverse reactions occurred in 59% of patients. Treatment was discontinued in 29% of patients and delayed in 65% of patients for an adverse reaction.
Serious adverse reactions reported in ≥4% of patients were pyrexia, diarrhea, anemia, increased AST, adrenal insufficiency, ascites, esophageal varices hemorrhage, hyponatremia, increased blood bilirubin, and pneumonitis.
Table 13 summarizes the adverse reactions and Table 14 summarizes the laboratory abnormalities of YERVOY in combination with nivolumab in CHECKMATE-040.
Table 13: Adverse Reactions Occurring in ≥10% of Patients Receiving YERVOY in Combination with Nivolumab in Cohort 4 of CHECKMATE-040 Adverse Reaction
YERVOY and Nivolumab
(n=49)
All Grades (%)
Grades 3-4 (%)
Skin and Subcutaneous Tissue
Rash
53
8
Pruritus
53
4
Musculoskeletal and Connective Tissue
Musculoskeletal pain
41
2
Arthralgia
10
0
Gastrointestinal
Diarrhea
39
4
Abdominal pain
22
6
Nausea
20
0
Ascites
14
6
Constipation
14
0
Dry mouth
12
0
Dyspepsia
12
2
Vomiting
12
2
Stomatitis
10
0
Respiratory, Thoracic and Mediastinal
Cough
37
0
Dyspnea
14
0
Pneumonitis
10
2
Metabolism and Nutrition
Decreased appetite
35
2
General
Fatigue
27
2
Pyrexia
27
0
Malaise
18
2
Edema
16
2
Influenza-like illness
14
0
Chills
10
0
Nervous System
Headache
22
0
Dizziness
20
0
Endocrine
Hypothyroidism
20
0
Adrenal insufficiency
18
4
Investigations
Weight decreased
20
0
Psychiatric
Insomnia
18
0
Blood and Lymphatic System
Anemia
10
4
Infections
Influenza
10
2
Vascular
Hypotension
10
0
Clinically important adverse reactions reported in <10% of patients receiving YERVOY with nivolumab were hyperglycemia (8%), colitis (4%), and increased blood creatine phosphokinase (2%).
Table 14: Select Laboratory Abnormalities (≥10%) Worsening from Baseline in Patients Receiving YERVOY in Combination with Nivolumab in Cohort 4 of CHECKMATE-040 Laboratory Abnormality
YERVOY and Nivolumab
(n=47)
All Grades (%)
Grades 3-4 (%)
Hematology
Lymphopenia
53
13
Anemia
43
4.3
Neutropenia
43
9
Leukopenia
40
2.1
Thrombocytopenia
34
4.3
Chemistry
Increased AST
66
40
Increased ALT
66
21
Increased bilirubin
55
11
Increased lipase
51
26
Hyponatremia
49
32
Hypocalcemia
47
0
Increased alkaline phosphatase
40
4.3
Increased amylase
38
15
Hypokalemia
26
2.1
Hyperkalemia
23
4.3
Increased creatinine
21
0
Hypomagnesemia
11
0
In patients who received YERVOY with nivolumab, virologic breakthrough occurred in 4 of 28 (14%) patients and 2 of 4 (50%) patients with active HBV or HCV at baseline, respectively. HBV virologic breakthrough was defined as at least a 1 log increase in HBV DNA for those patients with detectable HBV DNA at baseline. HCV virologic breakthrough was defined as a 1 log increase in HCV RNA from baseline.
First-line Treatment of Metastatic NSCLC: In Combination with Nivolumab
The safety of YERVOY in combination with nivolumab was evaluated in CHECKMATE-227, a randomized, multicenter, multi-cohort, open-label trial in patients with previously untreated metastatic or recurrent NSCLC with no EGFR or ALK genomic tumor aberrations [see Clinical Studies (14.6)]. The trial excluded patients with untreated brain metastases, carcinomatous meningitis, active autoimmune disease, or medical conditions requiring systemic immunosuppression. Patients received YERVOY 1 mg/kg by intravenous infusion over 30 minutes every 6 weeks and nivolumab 3 mg/kg by intravenous infusion over 30 minutes every 2 weeks or platinum-doublet chemotherapy every 3 weeks for 4 cycles. The median duration of therapy in YERVOY and nivolumab-treated patients was 4.2 months (range: 1 day to 25.5 months): 39% of patients received YERVOY and nivolumab for >6 months and 23% of patients received YERVOY and nivolumab for >1 year. The population characteristics were: median age 64 years (range: 26 to 87); 48% were ≥65 years of age, 76% White, and 67% male. Baseline ECOG performance status was 0 (35%) or 1 (65%), 85% were former/current smokers, 11% had brain metastases, 28% had squamous histology and 72% had non-squamous histology.
Serious adverse reactions occurred in 58% of patients. YERVOY and nivolumab were discontinued for adverse reactions in 24% of patients and 53% had at least one dose withheld for an adverse reaction.
The most frequent (≥2%) serious adverse reactions were pneumonia, diarrhea/colitis, pneumonitis, hepatitis, pulmonary embolism, adrenal insufficiency, and hypophysitis. Fatal adverse reactions occurred in 1.7% of patients; these included events of pneumonitis (4 patients), myocarditis, acute kidney injury, shock, hyperglycemia, multi-system organ failure, and renal failure. The most common (≥20%) adverse reactions were fatigue, rash, decreased appetite, musculoskeletal pain, diarrhea/colitis, dyspnea, cough, hepatitis, nausea, and pruritus.
Tables 15 and 16 summarize selected adverse reactions and laboratory abnormalities, respectively, in CHECKMATE-227.
Table 15: Adverse Reactions in ≥10% of Patients Receiving YERVOY and Nivolumab - CHECKMATE-227 a Includes fatigue and asthenia.
b Includes eyelid edema, face edema, generalized edema, localized edema, edema, edema peripheral, and periorbital edema.
c Includes autoimmune dermatitis, dermatitis, dermatitis acneiform, dermatitis allergic, dermatitis atopic, dermatitis bullous, dermatitis contact, dermatitis exfoliative, dermatitis psoriasiform, granulomatous dermatitis, rash generalized, drug eruption, dyshidrotic eczema, eczema, exfoliative rash, nodular rash, rash, rash erythematous, rash generalized, rash macular, rash maculo-papular, rash papular, rash pruritic, rash pustular, toxic skin eruption.
d Includes pruritus and pruritus generalized.
e Includes back pain, bone pain, musculoskeletal chest pain, musculoskeletal discomfort, musculoskeletal pain, myalgia, and pain in extremity.
f Includes colitis, colitis microscopic, colitis ulcerative, diarrhea, enteritis infectious, enterocolitis, enterocolitis infectious, and enterocolitis viral.
g Includes abdominal discomfort, abdominal pain, abdominal pain lower, abdominal pain upper, and abdominal tenderness.
h Includes dyspnea and dyspnea exertional.
i Includes cough and productive cough.
j Includes alanine aminotransferase increased, aspartate aminotransferase increased, autoimmune hepatitis, blood bilirubin increased, hepatic enzyme increased, hepatic failure, hepatic function abnormal, hepatitis, hepatitis E, hepatocellular injury, hepatotoxicity, hyperbilirubinemia, immune-mediated hepatitis, liver function test abnormal, liver function test increased, transaminases increased.
k Includes autoimmune thyroiditis, blood thyroid stimulating hormone increased, hypothyroidism, primary hypothyroidism, thyroiditis, and tri-iodothyronine free decreased.
l Contains blood thyroid stimulating hormone decreased, hyperthyroidism, and tri-iodothyronine free increased.
m Includes lower respiratory tract infection, lower respiratory tract infection bacterial, lung infection, pneumonia, pneumonia adenoviral, pneumonia aspiration, pneumonia bacterial, pneumonia klebsiella, pneumonia influenzal, pneumonia viral, atypical pneumonia, organizing pneumonia.Adverse Reaction
YERVOY and Nivolumab
(n=576)Platinum-doublet Chemotherapy
(n=570)All Grades
(%)Grades 3-4
(%)All Grades
(%)Grades 3-4
(%)General
Fatiguea
44
6
42
4.4
Pyrexia
18
0.5
11
0.4
Edemab
14
0.2
12
0.5
Skin and Subcutaneous Tissue
Rashc
34
4.7
10
0.4
Pruritusd
21
0.5
3.3
0
Metabolism and Nutrition
Decreased appetite
31
2.3
26
1.4
Musculoskeletal and Connective Tissue
Musculoskeletal paine
27
1.9
16
0.7
Arthralgia
13
0.9
2.5
0.2
Gastrointestinal
Diarrhea/colitisf
26
3.6
16
0.9
Nausea
21
1.0
42
2.5
Constipation
18
0.3
27
0.5
Vomiting
13
1.0
18
2.3
Abdominal paing
10
0.2
9
0.7
Respiratory, Thoracic, and Mediastinal
Dyspneah
26
4.3
16
2.1
Coughi
23
0.2
13
0
Hepatobiliary
Hepatitisj
21
9
10
1.2
Endocrine
Hypothyroidismk
16
0.5
1.2
0
Hyperthyroidisml
10
0
0.5
0
Infections and Infestations
Pneumoniam
13
7
8
4.0
Nervous System
Headache
11
0.5
6
0
Other clinically important adverse reactions in CHECKMATE-227 were:
Skin and Subcutaneous Tissue: urticaria, alopecia, erythema multiforme, vitiligo
Gastrointestinal: stomatitis, pancreatitis, gastritis
Musculoskeletal and Connective Tissue: arthritis, polymyalgia rheumatica, rhabdomyolysis
Nervous System: peripheral neuropathy, autoimmune encephalitis
Blood and Lymphatic System: eosinophilia
Eye Disorders: blurred vision, uveitis
Cardiac: atrial fibrillation, myocarditis
Table 16: Laboratory Values Worsening from Baselinea Occurring in ≥20% of Patients on YERVOY and Nivolumab - CHECKMATE-227 a Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: YERVOY and nivolumab group (range: 494 to 556 patients) and chemotherapy group (range: 469 to 542 patients). Laboratory Abnormality
YERVOY and Nivolumab
Platinum-doublet Chemotherapy
Grades 1-4
(%)Grades 3-4
(%)Grades 1-4
(%)Grades 3-4
(%)Hematology
Anemia
46
3.6
78
14
Lymphopenia
46
5
60
15
Chemistry
Hyponatremia
41
12
26
4.9
Increased AST
39
5
26
0.4
Increased ALT
36
7
27
0.7
Increased lipase
35
14
14
3.4
Increased alkaline phosphatase
34
3.8
20
0.2
Increased amylase
28
9
18
1.9
Hypocalcemia
28
1.7
17
1.3
Hyperkalemia
27
3.4
22
0.4
Increased creatinine
22
0.9
17
0.2
First-line Treatment of Metastatic or Recurrent NSCLC: In Combination with Nivolumab and Platinum-Doublet Chemotherapy
The safety of YERVOY in combination with nivolumab and platinum-doublet chemotherapy was evaluated in CHECKMATE-9LA [see Clinical Studies (14.6)]. Patients received either YERVOY 1 mg/kg administered every 6 weeks in combination with nivolumab 360 mg administered every 3 weeks and platinum-doublet chemotherapy administered every 3 weeks for 2 cycles; or platinum-doublet chemotherapy administered every 3 weeks for 4 cycles. The median duration of therapy in YERVOY in combination with nivolumab and platinum-doublet chemotherapy was 6 months (range: 1 day to 19 months): 50% of patients received YERVOY and nivolumab for >6 months and 13% of patients received YERVOY and nivolumab for >1 year.
Serious adverse reactions occurred in 57% of patients who were treated with YERVOY in combination with nivolumab and platinum-doublet chemotherapy. The most frequent (>2%) serious adverse reactions were pneumonia, diarrhea, febrile neutropenia, anemia, acute kidney injury, musculoskeletal pain, dyspnea, pneumonitis, and respiratory failure. Fatal adverse reactions occurred in 7 (2%) patients, and included hepatic toxicity, acute renal failure, sepsis, pneumonitis, diarrhea with hypokalemia, and massive hemoptysis in the setting of thrombocytopenia.
Study therapy with YERVOY in combination with nivolumab and platinum-doublet chemotherapy was permanently discontinued for adverse reactions in 24% of patients and 56% had at least one treatment withheld for an adverse reaction. The most common (>20%) adverse reactions were fatigue, musculoskeletal pain, nausea, diarrhea, rash, decreased appetite, constipation, and pruritus.
Tables 17 and 18 summarize selected adverse reactions and laboratory abnormalities, respectively, in CHECKMATE-9LA.
Table 17: Adverse Reactions in >10% of Patients Receiving YERVOY and Nivolumab and Platinum-Doublet Chemotherapy - CHECKMATE-9LA Toxicity was graded per NCI CTCAE v4.
a Includes fatigue and asthenia
b Includes myalgia, back pain, pain in extremity, musculoskeletal pain, bone pain, flank pain, muscle spasms, musculoskeletal chest pain, musculoskeletal disorder, osteitis, musculoskeletal stiffness, non-cardiac chest pain, arthralgia, arthritis, arthropathy, joint effusion, psoriatic arthropathy, synovitis
c Includes colitis, ulcerative colitis, diarrhea, and enterocolitis
d Includes abdominal discomfort, abdominal pain, lower abdominal pain, upper abdominal pain, and gastrointestinal pain
e Includes acne, dermatitis, acneiform dermatitis, allergic dermatitis, atopic dermatitis, bullous dermatitis, generalized exfoliative dermatitis, eczema, keratoderma blenorrhagica, palmar-plantar erythrodysaesthesia syndrome, rash, erythematous rash, generalized rash, macular rash, maculo-papular rash, morbilliform rash, papular rash, pruritic rash, skin exfoliation, skin reaction, skin toxicity, Stevens-Johnson syndrome, urticaria
f Includes pruritus and generalized pruritus
g Includes cough, productive cough, and upper-airway cough syndrome
h Includes dyspnea, dyspnea at rest, and exertional dyspnea
i Includes autoimmune thyroiditis, increased blood thyroid stimulating hormone, hypothyroidism, thyroiditis, and decreased free tri-iodothyronine
j Includes dizziness, vertigo and positional vertigoAdverse Reaction
YERVOY and Nivolumab and Platinum-Doublet Chemotherapy
(n=358)Platinum-Doublet Chemotherapy
(n=349)All Grades (%)
Grades 3-4 (%)
All Grades (%)
Grades 3-4 (%)
General
Fatiguea
49
5
40
4.9
Pyrexia
14
0.6
10
0.6
Musculoskeletal and Connective Tissue
Musculoskeletal painb
39
4.5
27
2.0
Gastrointestinal
Nausea
32
1.7
41
0.9
Diarrheac
31
6
18
1.7
Constipation
21
0.6
23
0.6
Vomiting
18
2.0
17
1.4
Abdominal paind
12
0.6
11
0.9
Skin and Subcutaneous Tissue
Rashe
30
4.7
10
0.3
Pruritusf
21
0.8
2.9
0
Alopecia
11
0.8
10
0.6
Metabolism and Nutrition
Decreased appetite
28
2.0
22
1.7
Respiratory, Thoracic and Mediastinal
Coughg
19
0.6
15
0.9
Dyspneah
18
4.7
14
3.2
Endocrine
Hypothyroidismi
19
0.3
3.4
0
Nervous System
Headache
11
0.6
7
0
Dizzinessj
11
0.6
6
0
Table 18: Laboratory Values Worsening from Baselinea Occurring in >20% of Patients on YERVOY and Nivolumab and Platinum-Doublet Chemotherapy - CHECKMATE-9LA a Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: YERVOY and nivolumab and platinum-doublet chemotherapy group (range: 197 to 347 patients) and platinum-doublet chemotherapy group (range: 191 to 335 patients). Laboratory Abnormality
YERVOY and Nivolumab and Platinum-Doublet Chemotherapy
Platinum-Doublet Chemotherapy
Grades 1-4 (%)
Grades 3-4 (%)
Grades 1-4 (%)
Grades 3-4 (%)
Hematology
Anemia
70
9
74
16
Lymphopenia
41
6
40
11
Neutropenia
40
15
42
15
Leukopenia
36
10
40
9
Thrombocytopenia
23
4.3
24
5
Chemistry
Hyperglycemia
45
7
42
2.6
Hyponatremia
37
10
27
7
Increased ALT
34
4.3
24
1.2
Increased lipase
31
12
10
2.2
Increased alkaline phosphatase
31
1.2
26
0.3
Increased amylase
30
7
19
1.3
Increased AST
30
3.5
22
0.3
Hypomagnesemia
29
1.2
33
0.6
Hypocalcemia
26
1.4
22
1.8
Increased creatinine
26
1.2
23
0.6
Hyperkalemia
22
1.7
21
2.1
First-line Treatment of Unresectable Malignant Pleural Mesothelioma: In Combination with Nivolumab
The safety of YERVOY in combination with nivolumab was evaluated in CHECKMATE-743, a randomized, open-label trial in patients with previously untreated unresectable malignant pleural mesothelioma [see Clinical Studies (14.7)]. Patients received either YERVOY 1 mg/kg over 30 minutes by intravenous infusion every 6 weeks and nivolumab 3 mg/kg over 30 minutes by intravenous infusion every 2 weeks for up to 2 years; or platinum-doublet chemotherapy for up to 6 cycles. The median duration of therapy in YERVOY and nivolumab-treated patients was 5.6 months (range: 0 to 26.2 months); 48% of patients received YERVOY and nivolumab for >6 months and 24% of patients received YERVOY and nivolumab for >1 year.
Serious adverse reactions occurred in 54% of patients who were treated with YERVOY in combination with nivolumab. The most frequent (≥2%) serious adverse reactions were pneumonia, pyrexia, diarrhea, pneumonitis, pleural effusion, dyspnea, acute kidney injury, infusion-related reaction, musculoskeletal pain, and pulmonary embolism. Fatal adverse reactions occurred in 4 (1.3%) patients and included pneumonitis, acute heart failure, sepsis, and encephalitis.
Both YERVOY and nivolumab were permanently discontinued due to adverse reactions in 23% of patients and 52% had at least one dose withheld due to an adverse reaction. An additional 4.7% of patients permanently discontinued YERVOY alone due to adverse reactions.
The most common (≥20%) adverse reactions were fatigue, musculoskeletal pain, rash, diarrhea, dyspnea, nausea, decreased appetite, cough, and pruritus.
Tables 19 and 20 summarize adverse reactions and laboratory abnormalities, respectively, in CHECKMATE-743.
Table 19: Adverse Reactions in ≥10% of Patients Receiving YERVOY and Nivolumab - CHECKMATE-743 a Includes fatigue and asthenia.
b Includes pyrexia and tumor-associated fever.
c Includes edema, generalized edema, peripheral edema, and peripheral swelling.
d Includes musculoskeletal pain, back pain, bone pain, flank pain, involuntary muscle contractions, muscle spasms, muscle twitching, musculoskeletal chest pain, musculoskeletal stiffness, myalgia, neck pain, non-cardiac chest pain, pain in extremity, polymyalgia rheumatica, and spinal pain.
e Includes rash, acne, acneiform dermatitis, allergic dermatitis, atopic dermatitis, autoimmune dermatitis, bullous dermatitis, contact dermatitis, dermatitis, drug eruption, dyshidrotic eczema, eczema, erythematous rash, exfoliative rash, generalized exfoliative dermatitis, generalized rash, granulomatous dermatitis, keratoderma blenorrhagica, macular rash, maculopapular rash, morbilliform rash, nodular rash, papular rash, psoriasiform dermatitis, pruritic rash, pustular rash, skin exfoliation, skin reaction, skin toxicity, Stevens-Johnson syndrome, toxic skin eruption, and urticaria.
f Includes pruritus, allergic pruritus, and generalized pruritus.
g Includes diarrhea, colitis, enteritis, infectious enteritis, enterocolitis, infectious enterocolitis, microscopic colitis, ulcerative colitis, and viral enterocolitis.
h Includes abdominal pain, abdominal discomfort, abdominal tenderness, gastrointestinal pain, lower abdominal pain, and upper abdominal pain.
i Includes dyspnea, dyspnea at rest, and exertional dyspnea.
j Includes cough, productive cough, and upper-airway cough syndrome.
k Includes hypothyroidism, autoimmune thyroiditis, decreased free tri-iodothyronine, increased blood thyroid stimulating hormone, primary hypothyroidism, thyroiditis, and autoimmune hypothyroidism.
l Includes upper respiratory tract infection, nasopharyngitis, pharyngitis, and rhinitis.
m Includes pneumonia, lower respiratory tract infection, lung infection, aspiration pneumonia, and Pneumocystis jirovecii pneumonia.Adverse Reaction
YERVOY and Nivolumab
(n=300)Chemotherapy
(n=284)All Grades
(%)Grades 3-4
(%)All Grades
(%)Grades 3-4
(%)General
Fatiguea
43
4.3
45
6
Pyrexiab
18
1.3
4.6
0.7
Edemac
17
0
8
0
Musculoskeletal and Connective Tissue
Musculoskeletal paind
38
3.3
17
1.1
Arthralgia
13
1.0
1.1
0
Skin and Subcutaneous Tissue
Rashe
34
2.7
11
0.4
Pruritusf
21
1.0
1.4
0
Gastrointestinal
Diarrheag
32
6
12
1.1
Nausea
24
0.7
43
2.5
Constipation
19
0.3
30
0.7
Abdominal painh
15
1
10
0.7
Vomiting
14
0
18
2.1
Respiratory, Thoracic, and Mediastinal
Dyspneai
27
2.3
16
3.2
Coughj
23
0.7
9
0
Metabolism and Nutrition
Decreased appetite
24
1.0
25
1.4
Endocrine
Hypothyroidismk
15
0
1.4
0
Infections and Infestations
Upper respiratory tract infectionl
12
0.3
7
0
Pneumoniam
10
4.0
4.2
2.1
Table 20: Laboratory Values Worsening from Baselinea Occurring in ≥20% of Patients on YERVOY and Nivolumab - CHECKMATE-743 a Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: YERVOY and nivolumab group (range: 109 to 297 patients) and chemotherapy group (range: 90 to 276 patients). Laboratory Abnormality
YERVOY and Nivolumab
Chemotherapy
Grades 1-4
(%)Grades 3-4
(%)Grades 1-4
(%)Grades 3-4
(%)Chemistry
Hyperglycemia
53
3.7
34
1.1
Increased AST
38
7
17
0
Increased ALT
37
7
15
0.4
Increased lipase
34
13
9
0.8
Hyponatremia
32
8
21
2.9
Increased alkaline phosphatase
31
3.1
12
0
Hyperkalemia
30
4.1
16
0.7
Hypocalcemia
28
0
16
0
Increased amylase
26
5
13
0.9
Increased creatinine
20
0.3
20
0.4
Hematology
Lymphopenia
43
8
57
14
Anemia
43
2.4
75
15
First-line Treatment of Unresectable Advanced or Metastatic ESCC: In Combination with Nivolumab
The safety of YERVOY in combination with nivolumab was evaluated in CHECKMATE-648, a randomized, active-controlled, multicenter, open-label trial in patients with previously untreated unresectable advanced, recurrent or metastatic ESCC [see Clinical Studies (14.8)]. Patients received one of the following treatments:
- •
- YERVOY 1 mg/kg every 6 weeks in combination with nivolumab 3 mg/kg every 2 weeks.
- •
- 5-FU (fluorouracil) 800 mg/m2/day intravenously on days 1 through 5 (for 5 days), and cisplatin 80 mg/m2 intravenously on day 1 (of a 4-week cycle).
Among patients who received YERVOY and nivolumab, the median duration of exposure was 2.8 months (range: 0 to 24 months).
Serious adverse reactions occurred in 69% of patients receiving YERVOY in combination with nivolumab.
The most frequent serious adverse reactions reported in ≥2% of patients who received YERVOY with nivolumab were pneumonia (10%), pyrexia (4.3%), pneumonitis (4.0%), aspiration pneumonia (3.7%), dysphagia (3.7%), hepatic function abnormal (2.8%), decreased appetite (2.8%), adrenal insufficiency (2.5%), and dehydration (2.5%).
Fatal adverse reactions occurred in 5 (1.6%) patients who received YERVOY in combination with nivolumab; these included pneumonitis, interstitial lung disease, pulmonary embolism, and acute respiratory distress syndrome.
Yervoy and/or nivolumab were discontinued in 23% of patients and delayed in 46% of patients for an adverse reaction.
The most common adverse reactions reported in ≥20% of patients treated with YERVOY in combination with nivolumab were rash, fatigue, pyrexia, nausea, diarrhea, and constipation.
Tables 21 and 22 summarize the adverse reactions and laboratory abnormalities, respectively, in CHECKMATE-648.
Table 21: Adverse Reactions in ≥10% of Patients Receiving YERVOY and Nivolumab - CHECKMATE-648 Adverse Reaction YERVOY and Nivolumab
(n=322)Cisplatin and 5-FU
(n=304)All Grades (%) Grades 3-4 (%) All Grades (%) Grades 3-4 (%) Toxicity was graded per NCI CTCAE v4.
a Includes dermatitis, dermatitis acneiform, dermatitis allergic, dermatitis bullous, drug eruption, exfoliative rash, rash erythematous, rash follicular, rash macular, rash maculo-papular, rash papular, and rash pruritic.
b Includes tumor associated fever.
c Includes asthenia and malaise.
d Includes aphthous ulcer, mouth ulceration, and mucosal inflammation.
e Includes abdominal discomfort, abdominal pain lower, and abdominal pain upper.
f Includes back pain, bone pain, musculoskeletal chest pain, myalgia, neck pain, pain in extremity, and spinal pain.
g Includes organizing pneumonia, pneumonia bacterial, and pneumonia pseudomonal.
h Includes productive cough.Skin and Subcutaneous Tissue
Rasha
31
3.1
7
0
Pruritis
17
0.9
3.6
0
General
Fatiguec
28
2.5
41
4.9
Pyrexiab
23
0.9
12
0.3
Gastrointestinal
Nausea
22
0.6
56
2.6
Diarrhea
22
1.9
20
2.0
Constipation
20
0.3
43
1.0
Vomiting
15
1.6
19
3.0
Dysphagia
12
5
12
4.9
Stomatitisd
11
0.6
35
3.0
Abdominal paine
10
0.9
11
0.7
Metabolism and Nutrition
Decreased appetite
17
4.0
50
6
Musculoskeletal and Connective Tissue
Musculoskeletal painf
14
0.6
8
0.3
Infections and Infestations
Pneumoniag
14
8
10
2.6
Endocrine
Hypothyroidism
14
0
0.3
0
Respiratory, Thoracic and Mediastinal
Coughh
13
0.3
13
0.3
Investigations
Weight decreased
12
1.9
11
1.0
Table 22: Laboratory Values Worsening from Baselinea Occurring in ≥10% of Patients on YERVOY and Nivolumab- CHECKMATE-648 Laboratory Abnormality
YERVOY and Nivolumab
(n=322)Cisplatin and 5-FU
(n=304)Grades 1-4 (%) Grades 3-4 (%) Grades 1-4 (%) Grades 3-4 (%) a Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: YERVOY and nivolumab group (range: 59 to 307 patients) or Cisplatin and 5-FU group (range: 56 to 283 patients). Hematology
Anemia
52
7
66
14
Lymphopenia
50
13
44
8
Neutropenia
13
1.3
48
13
Thrombocytopenia
12
1.0
29
2.8
Chemistry
Hyponatremia
45
11
40
8
Hyperglycemia
43
4.3
36
0.8
Increased AST
39
6
11
1.4
Increased ALT
33
6
8
0.7
Hypocalcemia
32
0
23
0.7
Increased alkaline phosphatase
31
3.3
15
0
Hyperkalemia
23
1.6
24
0.7
Hypokalemia
19
5
17
6
Hypercalcemia
15
2.0
8
0
Hypoglycemia
15
1.2
7
0
Increased creatinine
15
0.7
31
0.7
Hypomagnesemia
15
0
25
1.8
6.2 Immunogenicity
As with all therapeutic proteins, there is a potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies in the studies described below with the incidences of antibodies to other studies or to other products may be misleading.
Eleven (1.1%) of 1024 evaluable patients with unresectable or metastatic melanoma tested positive for treatment-emergent binding antibodies against ipilimumab in an electrochemiluminescent (ECL) based assay. This assay had substantial limitations in detecting anti-ipilimumab antibodies in the presence of ipilimumab. Seven (4.9%) of 144 patients receiving ipilimumab developed anti-ipilimumab antibodies and 7 (4.5%) of 156 patients receiving placebo for the adjuvant treatment of melanoma tested positive for anti-ipilimumab antibodies using an ECL assay with improved drug tolerance. No patients tested positive for neutralizing antibodies. No infusion-related reactions occurred in patients who tested positive for anti-ipilimumab antibodies.
Of the 499 patients evaluable for anti-ipilimumab antibodies in CHECKMATE-214 and CHECKMATE-142, 27 (5.4%) were positive for anti-ipilimumab antibodies; there were no patients with neutralizing antibodies against ipilimumab. There was no evidence of increased incidence of infusion reactions to YERVOY in patients with anti-ipilimumab antibodies.
Of 483 patients evaluable for anti-ipilimumab antibodies in CHECKMATE-227 Part 1, 8.5% were positive for treatment-emergent anti-ipilimumab antibodies. No patients had neutralizing antibodies against ipilimumab. In Part 1 of the same study, of 491 patients evaluable for anti-nivolumab antibodies, 36.7% were positive for anti-nivolumab antibodies and 1.4% had neutralizing antibodies against nivolumab.
Of 305 patients evaluable for anti-ipilimumab antibodies in CHECKMATE-9LA, 8% were positive for anti-ipilimumab antibodies and 1.6% were positive for anti-ipilimumab neutralizing antibodies. There was no evidence of increased incidence of infusion reactions to YERVOY in patients with anti-ipilimumab antibodies. Of 308 patients evaluable for anti-nivolumab antibodies in CHECKMATE-9LA, 34% were positive for anti-nivolumab antibodies and 2.6% had neutralizing antibodies against nivolumab.
Of 271 patients evaluable for anti-ipilimumab antibodies in CHECKMATE-743, 13.7% were positive for anti-ipilimumab antibodies and 0.4% were positive for anti-ipilimumab neutralizing antibodies. Of 269 patients evaluable for anti-nivolumab antibodies in CHECKMATE-743, 25.7% were positive for anti-nivolumab antibodies and 0.7% had neutralizing antibodies against nivolumab.
6.3 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of YERVOY. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and lymphatic system disorders: hemophagocytic lymphohistiocytosis (HLH)
Immune System: graft-versus-host disease, solid organ transplant rejection
Skin and Subcutaneous Tissue: Drug reaction with eosinophilia and systemic symptoms (DRESS syndrome)
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on findings from animal studies and its mechanism of action [see Clinical Pharmacology (12.1)], YERVOY can cause fetal harm when administered to a pregnant woman. There is insufficient human data for YERVOY exposure in pregnant women. In animal reproduction studies, administration of ipilimumab to cynomolgus monkeys from the onset of organogenesis through delivery resulted in higher incidences of abortion, stillbirth, premature delivery (with corresponding lower birth weight), and higher incidences of infant mortality in a dose-related manner (see Data). The effects of ipilimumab are likely to be greater during the second and third trimesters of pregnancy. Human IgG1 is known to cross the placental barrier and ipilimumab is an IgG1; therefore, ipilimumab has the potential to be transmitted from the mother to the developing fetus. Advise pregnant women of the potential risk to a fetus. Report pregnancies to Bristol-Myers Squibb at 1-844-593-7869.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
In a combined study of embryo-fetal and peri-postnatal development, pregnant cynomolgus monkeys received ipilimumab every 3 weeks from the onset of organogenesis in the first trimester through parturition. No treatment-related adverse effects on reproduction were detected during the first two trimesters of pregnancy. Beginning in the third trimester, administration of ipilimumab at doses resulting in exposures approximately 2.6 to 7.2 times the human exposure at a dose of 3 mg/kg resulted in dose-related increases in abortion, stillbirth, premature delivery (with corresponding lower birth weight), and an increased incidence of infant mortality. In addition, developmental abnormalities were identified in the urogenital system of 2 infant monkeys exposed in utero to 30 mg/kg of ipilimumab (7.2 times the humans exposure based on area under the curve at a dose of 3 mg/kg). One female infant monkey had unilateral renal agenesis of the left kidney and ureter, and 1 male infant monkey had an imperforate urethra with associated urinary obstruction and subcutaneous scrotal edema.
Genetically engineered mice heterozygous for CTLA-4 (CTLA-4+/−), the target for ipilimumab, appeared healthy and gave birth to healthy CTLA-4+/− heterozygous offspring. Mated CTLA-4+/− heterozygous mice also produced offspring deficient in CTLA-4 (homozygous negative, CTLA-4−/−). The CTLA-4−/− homozygous negative offspring appeared healthy at birth, exhibited signs of multiorgan lymphoproliferative disease by 2 weeks of age, and all died by 3 to 4 weeks of age with massive lymphoproliferation and multiorgan tissue destruction.
8.2 Lactation
Risk Summary
There are no data on the presence of YERVOY in human milk or its effects on the breastfed child or milk production. In monkeys, ipilimumab was present in milk (see Data). Because of the potential for serious adverse reactions in breastfed children, advise women not to breastfeed during treatment with YERVOY and for 3 months following the last dose.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Verify pregnancy status in females of reproductive potential prior to initiating YERVOY [see Use in Specific Populations (8.1)].
Contraception
YERVOY can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)]. Advise females of reproductive potential to use effective contraception during treatment with YERVOY and for 3 months following the last dose.
8.4 Pediatric Use
The safety and effectiveness of YERVOY have been established in pediatric patients aged 12 years and older for the following indications: as a single agent and in combination with nivolumab for unresectable or metastatic melanoma, and, in combination with nivolumab for MSI-H or dMMR mCRC that has progressed following treatment with a fluoropyrimidine, oxaliplatin, and irinotecan. Use of YERVOY for these indications is supported by evidence from adequate and well-controlled studies in adults with melanoma or MSI-H or dMMR mCRC and additional pharmacokinetic data in pediatric patients. Ipilimumab exposures in pediatric patients 12 years and older are comparable to that of adults, and the courses of melanoma and MSI-H or dMMR mCRC are similar in pediatric patients aged 12 years and older to that of adults to allow extrapolation of safety and efficacy [see Adverse Reactions (6.1), Clinical Pharmacology (12.3), Clinical Studies (14.4)].
The safety and effectiveness of YERVOY have not been established in pediatric patients younger than 12 years old with unresectable or metastatic melanoma or MSI-H or dMMR mCRC.
The safety and effectiveness of YERVOY have not been established in pediatric patients for the adjuvant treatment of melanoma or for the treatment of advanced renal cell carcinoma, hepatocellular carcinoma, metastatic non-small cell lung cancer, malignant pleural mesothelioma and esophageal cancer.
In a dose-finding trial (NCT01445379), 33 patients aged 2 to 21 years (median 13 years) with relapsed or refractory solid tumors were evaluated including unresectable stage IIIc or stage IV melanoma (12), progressive or refractory sarcomas (17), renal or bladder carcinoma (3), and neuroblastoma (1). No responses in the patients with non-melanoma solid tumors and no new safety signals were observed in pediatric patients in this study.
8.5 Geriatric Use
Single Agent
Of the 511 patients treated with YERVOY in Study MDX010-20 (unresectable or metastatic melanoma), 28% were 65 years and over. No overall differences in safety or effectiveness were observed between these patients and younger patients.
In Combination with Nivolumab
Of the 314 patients randomized to YERVOY administered with nivolumab in CHECKMATE-067, 41% were 65 years or older and 11% were 75 years or older. No overall differences in safety or effectiveness were reported between elderly patients and younger patients.
Of the 576 patients randomized to YERVOY 1 mg/kg every 6 weeks with nivolumab 3 mg/kg every 2 weeks in CHECKMATE-227 (NSCLC), 48% were 65 years or older and 10% were 75 years or older. No overall difference in safety was reported between older patients and younger patients; however, there was a higher discontinuation rate due to adverse reactions in patients aged 75 years or older (29%) relative to all patients who received YERVOY with nivolumab (18%). Of the 396 patients in the primary efficacy population (PD-L1 ≥1%) randomized to YERVOY 1 mg/kg every 6 weeks with nivolumab 3 mg/kg every 2 weeks in CHECKMATE-227, the hazard ratio for overall survival was 0.70 (95% CI: 0.55, 0.89) in the 199 patients younger than 65 years compared to 0.91 (95% CI: 0.72, 1.15) in the 197 patients 65 years or older [see Clinical Studies (14.6)].
Of the 303 patients randomized to YERVOY 1 mg/kg every 6 weeks in combination with nivolumab 3 mg/kg every 2 weeks in CHECKMATE-743 (malignant pleural mesothelioma), 77% were 65 years old or older and 26% were 75 years or older. No overall difference in safety was reported between older patients and younger patients; however, there were higher rates of serious adverse reactions and discontinuation rate due to adverse reactions in patients aged 75 years or older (68% and 35%, respectively) relative to all patients who received YERVOY with nivolumab (54% and 28%, respectively). For patients aged 75 years or older who received chemotherapy, the rate of serious adverse reactions was 34% and discontinuation due to adverse reactions was 26% relative to 28% and 19% respectively for all patients. The hazard ratio for overall survival was 0.76 (95% CI: 0.52, 1.11) in the 71 patients younger than 65 years compared to 0.74 (95% CI: 0.59, 0.93) in the 232 patients 65 years or older randomized to YERVOY in combination with nivolumab.
Of the 550 patients randomized to YERVOY 1 mg/kg with nivolumab in CHECKMATE-214 (renal cell carcinoma), 38% were 65 years or older and 8% were 75 years or older. No overall difference in safety was observed between these patients and younger patients. In geriatric patients with intermediate or poor risk, no overall difference in effectiveness was observed.
Of the 49 patients who received YERVOY 3 mg/kg with nivolumab in Cohort 4 of CHECKMATE-040 (hepatocellular carcinoma), 29% were between 65 years and 74 years of age and 8% were 75 years or older. Clinical studies of YERVOY in combination with nivolumab did not include sufficient numbers of patients with hepatocellular carcinoma aged 65 and over to determine whether they respond differently from younger patients.
Of the 325 patients who received YERVOY 1 mg/kg every 6 weeks in combination with nivolumab 3 mg/kg every 2 weeks in CHECKMATE-648 (ESCC), 43% were 65 years old or older and 7% were 75 years or older. No overall difference in safety was reported between older patients and younger patients; however, there was a higher discontinuation rate due to adverse reactions in patients aged 75 years or older (38%) relative to all patients who received YERVOY with nivolumab (23%). For patients aged 75 years or older who received chemotherapy, the discontinuation rate due to adverse reactions was 33% relative to 23% for all patients.
Study CA184-029 (adjuvant treatment of melanoma) and CHECKMATE-142 (metastatic colorectal cancer) did not include sufficient numbers of patients aged 65 years and older to determine whether they respond differently from younger patients.
In Combination with Nivolumab and Platinum-Doublet Chemotherapy
Of the 361 patients randomized to YERVOY 1 mg/kg every 6 weeks in combination with nivolumab 360 mg every 3 weeks and platinum-doublet chemotherapy every 3 weeks (for 2 cycles) in CHECKMATE-9LA (NSCLC), 51% were 65 years or older and 10% were 75 years or older. No overall difference in safety was reported between older patients and younger patients; however, there was a higher discontinuation rate due to adverse reactions in patients aged 75 years or older (43%) relative to all patients who received YERVOY with nivolumab and chemotherapy (24%). For patients aged 75 years or older who received chemotherapy only, the discontinuation rate due to adverse reactions was 16% relative to all patients who had a discontinuation rate of 13%. Based on an updated analysis for overall survival, of the 361 patients randomized to YERVOY in combination with nivolumab and platinum-doublet chemotherapy in CHECKMATE-9LA, the hazard ratio for overall survival was 0.61 (95% CI: 0.47, 0.80) in the 176 patients younger than 65 years compared to 0.73 (95% CI: 0.56, 0.95) in the 185 patients 65 years or older.
-
11 DESCRIPTION
Ipilimumab is a human cytotoxic T-lymphocyte antigen 4 (CTLA-4)-blocking antibody. Ipilimumab is a recombinant IgG1 kappa immunoglobulin with an approximate molecular weight of 148 kDa. Ipilimumab is produced in mammalian (Chinese hamster ovary) cell culture.
YERVOY (ipilimumab) injection, for intravenous use is a sterile, preservative-free, clear to slightly opalescent, colorless to pale-yellow solution, which may contain a small amount of visible translucent-to-white, amorphous ipilimumab particulates. It is supplied in single-dose vials of 50 mg/10 mL or 200 mg/40 mL. Each milliliter contains 5 mg of ipilimumab and the following inactive ingredients: diethylene triamine pentaacetic acid (DTPA) (0.04 mg), mannitol (10 mg), polysorbate 80 (vegetable origin) (0.1 mg), sodium chloride (5.85 mg), tris hydrochloride (3.15 mg), and Water for Injection, USP at a pH of 7.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
CTLA-4 is a negative regulator of T-cell activity. Ipilimumab is a monoclonal antibody that binds to CTLA-4 and blocks the interaction of CTLA-4 with its ligands, CD80/CD86. Blockade of CTLA-4 has been shown to augment T-cell activation and proliferation, including the activation and proliferation of tumor infiltrating T-effector cells. Inhibition of CTLA-4 signaling can also reduce T-regulatory cell function, which may contribute to a general increase in T cell responsiveness, including the anti-tumor immune response.
12.3 Pharmacokinetics
The pharmacokinetics (PK) of ipilimumab was studied in 785 patients with unresectable or metastatic melanoma who received doses of 0.3, 3, or 10 mg/kg once every 3 weeks for 4 doses. The PK of ipilimumab is linear in the dose range of 0.3 mg/kg to 10 mg/kg. Following administration of YERVOY every 3 weeks, the systemic accumulation was 1.5-fold or less. Steady-state concentrations of ipilimumab were reached by the third dose; the mean minimum concentration (Cmin) at steady state was 19.4 mcg/mL at 3 mg/kg and 58.1 mcg/mL at 10 mg/kg every 3 weeks.
Elimination
The mean (percent coefficient of variation) terminal half-life (t1/2) was 15.4 days (34%) and then mean (percent coefficient of variation) clearance (CL) was 16.8 mL/h (38%).
The CL of ipilimumab was unchanged in presence of anti-ipilimumab antibodies.
Specific Populations
The CL of ipilimumab increased with increasing body weight supporting the recommended body weight (mg/kg) based dosing. The following factors had no clinically important effect on the CL of ipilimumab: age (range: 23 to 88 years), sex, performance status, renal impairment (glomerular filtration rate ≥15 mL/min/1.73 m2), mild hepatic impairment (total bilirubin [TB] >1 to 1.5 times the upper limit of normal [ULN] or AST > ULN), previous cancer therapy, and baseline lactate dehydrogenase (LDH) levels. The effect of race was not examined due to limited data available in non-White racial groups. YERVOY has not been studied in patients with moderate (TB > 1.5 to 3 times ULN and any AST) or severe (TB >3 times ULN and any AST) hepatic impairment.
Pediatric Patients
The exposures of ipilimumab in pediatric patients 12 years and older are comparable to those in adult patients at the recommended dosage.
Drug Interaction Studies
Ipilimumab with Nivolumab
When YERVOY 1 mg/kg was administered with nivolumab 3 mg/kg every 3 weeks, the CL of ipilimumab was unchanged compared to when YERVOY was administered alone.
When YERVOY 3 mg/kg every 3 weeks was administered in combination with nivolumab 1 mg/kg every 3 weeks, the CL of ipilimumab was unchanged compared to ipilimumab administered alone and the CL of nivolumab was increased by 29% compared to nivolumab administered alone.
When YERVOY 1 mg/kg every 6 weeks was administered in combination with nivolumab 3 mg/kg every 2 weeks, the CL of ipilimumab increased by 30% compared to YERVOY administered alone and the CL of nivolumab was unchanged compared to nivolumab administered alone.
When YERVOY 1 mg/kg every 6 weeks was administered in combination with nivolumab 360 mg every 3 weeks and chemotherapy, the CL of ipilimumab increased by 22% compared to YERVOY administered alone and the CL of nivolumab was unchanged compared to nivolumab administered alone.
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
14.1 Unresectable or Metastatic Melanoma
The efficacy of YERVOY were investigated in a Study MDX010-20, a randomized (3:1:1), double-blind, double-dummy trial (NCT00094653) that included patients with unresectable or metastatic melanoma previously treated with one or more of the following: aldesleukin, dacarbazine, temozolomide, fotemustine, or carboplatin. The trial enrolled only patients with HLA-A2*0201 genotype; this HLA genotype facilitates the immune presentation of the investigational peptide vaccine. The trial excluded patients with active autoimmune disease or those receiving systemic immunosuppression for organ transplantation. Patients were randomized to YERVOY administered at a dose of 3 mg/kg as an intravenous infusion every 3 weeks for 4 doses with an investigational peptide vaccine with incomplete Freund’s adjuvant - gp100 administered at a dose of 2 mg peptide by deep subcutaneous injection every 3 weeks for 4 doses; gp100 administered at a dose of 2 mg by deep subcutaneous injection every 3 weeks for 4 doses as a single agent with a placebo; or YERVOY administered at a dose of 3 mg/kg by intravenous infusion every 3 weeks for 4 doses with a placebo. The major efficacy outcome measure was overall survival (OS) in the YERVOY and gp100 arm compared to that in the single-agent gp100 arm. Secondary efficacy outcome measures were OS in the YERVOY and gp100 arm compared to the YERVOY arm, OS in the YERVOY arm compared to the gp100 arm, best overall response rate (BORR) as assessed by the investigator at week 24 between each of the trial arms, and duration of response. Assessment of tumor response was conducted at weeks 12 and 24, and every 3 months thereafter. Patients with evidence of objective tumor response at 12 or 24 weeks had assessment for confirmation of durability of response at 16 or 28 weeks, respectively.
A total of 676 patients were randomized, 403 to YERVOY and gp100 arm, 137 to YERVOY single agent arm and 136 to gp100 single agent arm. Of the randomized patients, 61%, 59%, and 54% in the YERVOY and gp100, YERVOY, and gp100 arms, respectively, were male. Twenty-nine percent were ≥ 65 years of age, the median age was 57 years, 71% had M1c stage, 12% had a history of previously treated brain metastasis, 98% had ECOG performance status of 0 and 1, 23% had received aldesleukin, and 38% had elevated LDH level. Sixty-one percent of patients randomized to either YERVOY-containing arm received all 4 planned doses. The median duration of follow-up was 8.9 months.
The efficacy results are shown in Table 23 and Figure 1.
Table 23: Efficacy Results for Study MDX010-20 a Not adjusted for multiple comparisons
b Not reachedYERVOY 3 mg/kg
n=137
YERVOY 3 mg/kg and gp100
n=403gp100
n=136
Overall Survival
Median in months (95% CI)
10 (8.0, 13.8)
10 (8.5, 11.5)
6 (5.5, 8.7)
Hazard ratio (vs. gp100) (95% CI)
0.66 (0.51, 0.87)
0.68 (0.55, 0.85)
p-value
p=0.0026a
p=0.0004
Hazard ratio (vs. YERVOY) (95% CI)
1.04 (0.83, 1.30)
Best Overall Response Rate (BORR)
(95% CI)
10.9%
(6.3%, 17.4%)
5.7%
(3.7%, 8.4%)
1.5%
(0.2%, 5.2%)
Median duration of response in months
NRb
11.5
NRb
Figure 1: Kaplan Meier Curves for Overall Survival in Study MDX010-20
Previously Untreated Metastatic Melanoma: In Combination with Nivolumab
CHECKMATE-067 (NCT01844505) was a multicenter, randomized (1:1:1), double-blind trial in which 945 patients with previously untreated, unresectable or metastatic melanoma were randomized to one of the following arms: YERVOY and nivolumab, nivolumab, or YERVOY. Patients were required to have completed adjuvant or neoadjuvant treatment at least 6 weeks prior to randomization and have no prior treatment with anti-CTLA-4 antibody and no evidence of active brain metastasis, ocular melanoma, autoimmune disease, or medical conditions requiring systemic immunosuppression.
Patients were randomized to receive:
- •
- YERVOY 3 mg/kg with nivolumab 1 mg/kg intravenously every 3 weeks for 4 doses, followed by nivolumab as a single agent at a dose of 3 mg/kg by intravenous infusion every 2 weeks (YERVOY and nivolumab arm),
- •
- Nivolumab 3 mg/kg by intravenous infusion every 2 weeks (nivolumab arm), or
- •
- YERVOY 3 mg/kg intravenously every 3 weeks for 4 doses followed by placebo every 2 weeks (YERVOY arm)
Randomization was stratified by PD-L1 expression (≥5% vs. <5% tumor cell membrane expression) as determined by a clinical trial assay, BRAF V600 mutation status, and M stage per the AJCC staging system (M0, M1a, M1b vs. M1c). Tumor assessments were conducted 12 weeks after randomization then every 6 weeks for the first year, and every 12 weeks thereafter. The major efficacy outcome measures were investigator-assessed PFS per RECIST v1.1 and OS. Additional efficacy outcome measures were confirmed ORR and duration of response.
The trial population characteristics were: median age 61 years (range: 18 to 90); 65% male; 97% White; ECOG performance score 0 (73%) or 1 (27%). Disease characteristics were: AJCC Stage IV disease (93%); M1c disease (58%); elevated LDH (36%); history of brain metastases (4%); BRAF V600 mutation-positive melanoma (32%); PD-L1 ≥5% tumor cell membrane expression as determined by the clinical trials assay (46%); and prior adjuvant therapy (22%).
CHECKMATE-067 demonstrated statistically significant improvements in OS and PFS for patients randomized to either nivolumab-containing arm as compared with the YERVOY arm. The trial was not designed to assess whether adding YERVOY to nivolumab improves PFS or OS compared to nivolumab as a single agent. Efficacy results are shown in Table 24 and Figure 2.
Table 24: Efficacy Results - CHECKMATE-067 a OS results are based on final OS analysis with 28 months of minimum follow-up; PFS (co-primary endpoint) and ORR (secondary endpoint) results were based on primary analysis with 9 months of minimum follow-up.
b Based on a stratified proportional hazards model.
c Based on stratified log-rank test.
d If the maximum of the two OS p-values is less than 0.04 (a significance level assigned by the Hochberg procedure), then both p-values are considered significant.
e p-value is compared with .005 of the allocated alpha for final PFS treatment comparisons.
f Based on the stratified Cochran-Mantel-Haenszel test.
+ Censored observationYERVOY and
Nivolumab
(n=314)Nivolumab
(n=316)YERVOY
(n=315)Overall Survivala
Deaths (%)
128 (41)
142 (45)
197 (63)
Hazard ratiob (vs. YERVOY)
(95% CI)
0.55
(0.44, 0.69)
0.63
(0.50, 0.78)
p-valuec, d
<0.0001
<0.0001
Progression-free Survivala
Disease progression or death
151 (48%)
174 (55%)
234 (74%)
Median (months)
(95% CI)
11.5
(8.9, 16.7)6.9
(4.3, 9.5)2.9
(2.8, 3.4)Hazard ratiob (vs. YERVOY)
(95% CI)
0.42
(0.34, 0.51)
0.57
(0.47, 0.69)
p-valuec, e
<0.0001
<0.0001
Confirmed Overall Response Ratea
50%
40%
14%
(95% CI)
(44, 55)
(34, 46)
(10, 18)
p-valuef
<0.0001
<0.0001
Complete response
8.9%
8.5%
1.9%
Partial response
41%
31%
12%
Duration of Response
Proportion ≥6 months in duration
76%
74%
63%
Range (months)
1.2+ to 15.8+
1.3+ to 14.6+
1.0+ to 13.8+
Figure 2: Overall Survival - CHECKMATE-067
Based on a minimum follow-up of 48 months, the median OS was not reached (95% CI: 38.2, NR) in the YERVOY and nivolumab arm. The median OS was 36.9 months (95% CI: 28.3, NR) in the nivolumab arm and 19.9 months (95% CI: 16.9, 24.6) in the YERVOY arm.
Based on a minimum follow-up of 28 months, the median PFS was 11.7 months (95% CI: 8.9, 21.9) in the YERVOY and nivolumab arm, 6.9 months (95% CI: 4.3, 9.5) in the nivolumab arm, and 2.9 months (95% CI: 2.8, 3.2) in the YERVOY arm. Based on a minimum follow-up of 28 months, the proportion of responses lasting ≥24 months was 55% in the YERVOY and nivolumab arm, 56% in the nivolumab arm, and 39% in the YERVOY arm.
14.2 Adjuvant Treatment of Melanoma
The efficacy of YERVOY for the adjuvant treatment of melanoma was evaluated in Study CA184-029 (NCT00636168), a randomized (1:1), double-blind, placebo-controlled trial in patients with resected Stage IIIA (>1 mm nodal involvement), IIIB, and IIIC (with no in-transit metastases) histologically confirmed cutaneous melanoma. Enrollment required complete resection of melanoma with full lymphadenectomy within 12 weeks prior to randomization. Patients with prior therapy for melanoma, autoimmune disease, and prior or concomitant use of immunosuppressive agents were ineligible. Patients were randomized to receive YERVOY 10 mg/kg or placebo as an intravenous infusion every 3 weeks for 4 doses, followed by YERVOY 10 mg/kg or placebo every 12 weeks from Week 24 to Week 156 (3 years) or until documented disease recurrence or unacceptable toxicity. Randomization was stratified by stage according to American Joint Committee on Cancer (AJCC) 2002 classification (Stage IIIA >1 mm nodal involvement, Stage IIIB, Stage IIIC with 1 to 3 involved lymph nodes, and Stage IIIC with ≥4 involved lymph nodes) and by region (North America, Europe, and Australia). The major efficacy outcome measures were independent review committee (IRC)-assessed recurrence-free survival (RFS), defined as the time between the date of randomization and the earliest date of first recurrence (local, regional, or distant metastasis) or death, and overall survival. Tumor assessment was conducted every 12 weeks for the first 3 years then every 24 weeks until distant recurrence.
Among 951 patients enrolled, 475 were randomized to receive YERVOY and 476 to placebo. Median age was 51 years (range: 18 to 84), 62% were male, 99% were White, 94% had ECOG performance status of 0. With regard to disease stage, 20% had Stage IIIA with lymph nodes >1 mm, 44% had Stage IIIB, and 36% had Stage IIIC (with no in-transit metastases). Other disease characteristics of the trial population were: clinically palpable lymph nodes (58%), 2 or more positive lymph nodes (54%), and ulcerated primary lesions (42%).
The efficacy results are in Table 25 and Figure 3.
Table 25: Efficacy Results for Study CA184-029 a Stratified by disease stage. YERVOY 10 mg/kg
n=475Placebo
n=476Recurrence-Free Survival
Number of events
234 (49%)
294 (62%)
Recurrence
220
289
Death
14
5
Median in months (95% CI)
26 (19, 39)
17 (13, 22)
Hazard ratio (95% CI)
0.75 (0.64, 0.90)
p-value (stratified log-ranka)
p<0.002
Overall Survival
Number of deaths
162 (34%)
214 (45%)
Hazard ratio (95% CI)
0.72 (0.58, 0.88)
p-value (stratified log-ranka)
p<0.002
Figure 3: Kaplan-Meier Curves for Overall Survival in Study CA184-029
14.3 Advanced Renal Cell Carcinoma
The efficacy of YERVOY with nivolumab was evaluated in CHECKMATE-214 (NCT02231749), a randomized (1:1), open-label study in patients with previously untreated advanced RCC. Patients were included regardless of their PD-L1 status. CHECKMATE-214 excluded patients with any history of or concurrent brain metastases, active autoimmune disease, or medical conditions requiring systemic immunosuppression. Patients were randomized to nivolumab 3 mg/kg and YERVOY 1 mg/kg administered intravenously every 3 weeks for 4 doses followed by nivolumab 3 mg/kg every two weeks or to sunitinib administered orally 50 mg daily for the first 4 weeks of each 6-week cycle. Treatment continued until disease progression or unacceptable toxicity. Patients were stratified by International Metastatic RCC Database Consortium (IMDC) prognostic score and region. The major efficacy outcome measures were OS, PFS (IRRC-assessed), and confirmed ORR (IRRC-assessed) in intermediate/poor risk patients. Intermediate/poor risk patients had at least 1 or more of 6 prognostic risk factors as per the IMDC criteria: less than one year from time of initial RCC diagnosis to randomization, Karnofsky performance status (KPS) <80%, hemoglobin less than the lower limit of normal, corrected calcium >10 mg/dL, platelet count > ULN, and absolute neutrophil count > ULN.
A total of 847 patients were randomized, 425 to YERVOY with nivolumab and 422 to sunitinib. The median age was 61 years (range: 21 to 85) with 38% ≥65 years of age and 8% ≥75 years of age. The majority of patients were male (73%) and White (87%) and 26% and 74% of patients had a baseline KPS of 70% to 80% and 90% to 100%, respectively.
Efficacy results from CHECKMATE-214 are presented in Table 26 and Figure 4. In intermediate/poor risk patients, the trial demonstrated statistically significant improvement in OS and ORR for patients randomized to YERVOY and nivolumab arm as compared with sunitinib arm. OS benefit was observed regardless of PD-L1 expression level. The trial did not demonstrate a statistically significant improvement in PFS.
Table 26: Efficacy Results for CHECKMATE-214 a Based on a stratified proportional hazards model.
b Based on a stratified log-rank test.
c p-value is compared to alpha 0.002 in order to achieve statistical significance.
d Based on the stratified DerSimonian-Laird test.
e p-value is compared to alpha 0.001 in order to achieve statistical significance.
f Not Significant at alpha level of 0.009Efficacy Parameter
Intermediate/Poor-Risk
YERVOY 1 mg/kg and Nivolumab
n=425Sunitinib
n=422Overall Survival
Number of deaths
140 (32.9%)
188 (44.5%)
Median in months
NE
25.9
Hazard ratio (99.8% CI)a
0.63 (0.44, 0.89)
p-valueb,c
<0.0001
Confirmed Objective Response Rate (95% CI)
41.6% (36.9%, 46.5%)
26.5% (22.4%, 31.0%)
Complete Response
40 (9.4%)
5 (1.2%)
Partial Response
137 (32.2%)
107 (25.4%)
Median duration of response in months (95% CI)
NE (21.8, NE)
18.2 (14.8, NE)
p-valued,e
<0.0001
Progression-free Survival
Number of events (progression or death)
228 (53.6%)
228 (54.0%)
Median in months
11.6
8.4
Hazard ratio (99.1% CI)a
0.82 (0.64, 1.05)
p-valueb
NSf
Figure 4: Kaplan-Meier Curves for Overall Survival (Intermediate/Poor Risk Population) in CHECKMATE-214
CHECKMATE-214 also randomized 249 favorable risk patients as per IMDC criteria to nivolumab and YERVOY (n=125) or to sunitinib (n=124). These patients were not evaluated as part of the efficacy analysis population. OS in favorable risk patients receiving nivolumab and YERVOY compared to sunitinib has a hazard ratio of 1.45 (95% CI: 0.75, 2.81). The efficacy of nivolumab and YERVOY in previously untreated renal cell carcinoma with favorable risk disease has not been established.
14.4 Microsatellite Instability-High or Mismatch Repair Deficient Metastatic Colorectal Cancer
The efficacy of YERVOY with nivolumab was evaluated in CHECKMATE-142 (NCT02060188), a multicenter, non-randomized, multiple parallel-cohort, open-label study conducted in patients with locally determined dMMR or MSI-H mCRC who had disease progression during or after prior treatment with fluoropyrimidine-, oxaliplatin-, or irinotecan-based chemotherapy. Key eligibility criteria were at least one prior line of treatment for metastatic disease, ECOG PS 0 or 1, and absence of the following: active brain metastases, active autoimmune disease, or medical conditions requiring systemic immunosuppression. Patients enrolled in the YERVOY and nivolumab MSI-H or dMMR mCRC cohort received YERVOY 1 mg/kg and nivolumab 3 mg/kg intravenously every 3 weeks for 4 doses, followed by nivolumab 3 mg/kg intravenously as a single agent every 2 weeks. Efficacy outcome measures were overall response rate (ORR) as assessed by Blinded Independent Central Review (BICR) using Response Evaluation Criteria in Solid Tumors (RECIST v1.1) and duration of response (DOR). Tumor assessments were conducted every 6 weeks for the first 24 weeks and every 12 weeks thereafter.
A total of 119 patients were enrolled in the YERVOY and nivolumab cohort. The median age was 58 years (range: 21 to 88), with 32% ≥65 years of age and 9% ≥75 years of age; 59% were male and 92% were white. Baseline ECOG PS was 0 (45%) or 1 (55%), and 29% were reported to have Lynch Syndrome. Across the cohort, 69% received prior treatment with a fluoropyrimidine, oxaliplatin, and irinotecan; 10%, 40%, 24%, and 15% received 1, 2, 3, or ≥4 prior lines of therapy for metastatic disease, respectively, and 29% had received an anti-EGFR antibody.
Efficacy results are shown in Table 27.
Table 27: Efficacy Results in MSI-H/dMMR Cohort of CHECKMATE-142 a Minimum follow-up 27.5 months for all patients treated with YERVOY and nivolumab (n=119).
b Estimated using the Clopper-Pearson method.YERVOY and Nivolumaba
MSI-H/dMMR CohortAll Patients
(n=119)Prior Treatment (Fluoropyrimidine, Oxaliplatin, and Irinotecan)
(n=82)Overall Response Rate per BICR; n (%)
71 (60%)
46 (56%)
(95% CI)b
(50, 69)
(45, 67)
Complete Response (%)
17 (14%)
11 (13%)
Partial Response (%)
54 (45%)
35 (43%)
Duration of Response
Proportion of responders with ≥6 months response duration
89%
87%
Proportion of responders with ≥12 months response duration
77%
74%
14.5 Hepatocellular Carcinoma
CHECKMATE-040 (NCT01658878) was a multicenter, multiple cohort, open-label trial conducted in patients with HCC who progressed on or were intolerant to sorafenib. Additional eligibility criteria included histologic confirmation of HCC and Child-Pugh Class A cirrhosis. The trial excluded patients with active autoimmune disease, brain metastasis, a history of hepatic encephalopathy, clinically significant ascites, infection with HIV, or active co-infection with hepatitis B virus (HBV) and hepatitis C virus (HCV) or HBV and hepatitis D virus (HDV); however, patients with only active HBV or HCV were eligible.
The efficacy of YERVOY 3 mg/kg in combination with nivolumab 1 mg/kg was evaluated in Cohort 4 of CHECKMATE-040. A total of 49 patients received the combination regimen, which was administered every 3 weeks for four doses, followed by single-agent nivolumab at 240 mg every 2 weeks until disease progression or unacceptable toxicity.
The median age was 60 years (range: 18 to 80); 88% were male; 74% were Asian, and 25% were White. Baseline ECOG performance status was 0 (61%) or 1 (39%). Fifty-seven percent (57%) of patients had active HBV infection, 8% had active HCV infection, and 35% had no evidence of active HBV or HCV. The etiology for HCC was alcoholic liver disease in 16% and non-alcoholic liver disease in 6% of patients. Child-Pugh class and score was A5 for 82% and A6 for 18%; 80% of patients had extrahepatic spread; 35% had vascular invasion; and 51% had alfa-fetoprotein (AFP) levels ≥400 µg/L. Prior treatment history included surgery (74%), radiotherapy (29%), or local treatment (59%). All patients had received prior sorafenib, of whom 10% were unable to tolerate sorafenib; 29% of patients had received 2 or more prior systemic therapies.
Efficacy results are shown in Table 28.
Table 28: Efficacy Results - Cohort 4 of CHECKMATE-040 a Confirmed by BICR.
b Confidence interval is based on the Clopper and Pearson method.YERVOY and Nivolumab
(Cohort 4)
(n=49)Overall Response Rate per BICR,a n (%), RECIST v1.1
16 (33%)
(95% CI)b
(20, 48)
Complete response
4 (8%)
Partial response
12 (24%)
Duration of Response per BICR,a RECIST v1.1
n=16
Range (months)
4.6, 30.5+
Percent with duration ≥6 months
88%
Percent with duration ≥12 months
56%
Percent with duration ≥24 months
31%
Overall Response Rate per BICR,a n (%), mRECIST
17 (35%)
(95% CI)b
(22, 50)
Complete response
6 (12%)
Partial response
11 (22%)
14.6 Metastatic Non-Small Cell Lung Cancer
First-line Treatment of Metastatic Non-Small Cell Lung Cancer (NSCLC) Expressing PD-L1 (≥1%): In Combination with Nivolumab
CHECKMATE-227 (NCT02477826) was a randomized, open-label, multi-part trial in patients with metastatic or recurrent NSCLC. The study included patients (18 years of age or older) with histologically confirmed Stage IV or recurrent NSCLC (per the 7th International Association for the Study of Lung Cancer [ASLC] classification), ECOG performance status 0 or 1, and no prior anticancer therapy. Patients were enrolled regardless of their tumor PD-L1 status. Patients with known EGFR mutations or ALK translocations sensitive to available targeted inhibitor therapy, untreated brain metastases, carcinomatous meningitis, active autoimmune disease, or medical conditions requiring systemic immunosuppression were excluded from the study. Patients with treated brain metastases were eligible if neurologically returned to baseline at least 2 weeks prior to enrolment, and either off corticosteroids, or on a stable or decreasing dose of <10 mg daily prednisone equivalents.
Primary efficacy results were based on Part 1a of the study, which was limited to patients with PD-L1 tumor expression ≥1%. Tumor specimens were evaluated prospectively using the PD-L1 IHC 28-8 pharmDx assay at a central laboratory. Randomization was stratified by tumor histology (non-squamous versus squamous). The evaluation of efficacy relied on the comparison between:
- •
- YERVOY 1 mg/kg administered intravenously over 30 minutes every 6 weeks in combination with nivolumab 3 mg/kg administered intravenously over 30 minutes every 2 weeks; or
- •
- Platinum-doublet chemotherapy
Chemotherapy regimens consisted of pemetrexed (500 mg/m2) and cisplatin (75 mg/m2) or pemetrexed (500 mg/m2) and carboplatin (AUC 5 or 6) for non-squamous NSCLC or gemcitabine (1000 or 1250 mg/m2) and cisplatin (75 mg/m2) or gemcitabine (1000 mg/m2) and carboplatin (AUC 5) (gemcitabine was administered on Days 1 and 8 of each cycle) for squamous NSCLC.
Study treatment continued until disease progression, unacceptable toxicity, or for up to 24 months. Treatment continued beyond disease progression if a patient was clinically stable and was considered to be deriving clinical benefit by the investigator. Patients who discontinued combination therapy because of an adverse event attributed to YERVOY were permitted to continue nivolumab as a single agent. Tumor assessments were performed every 6 weeks from the first dose of study treatment for the first 12 months, then every 12 weeks until disease progression or study treatment was discontinued. The primary efficacy outcome measure was OS. Additional efficacy outcome measures included PFS, ORR, and duration of response as assessed by BICR.
In Part 1a, a total of 793 patients were randomized to receive either YERVOY in combination with nivolumab (n=396) or platinum-doublet chemotherapy (n=397). The median age was 64 years (range: 26 to 87) with 49% of patients ≥65 years and 10% of patients ≥75 years, 76% White, and 65% male. Baseline ECOG performance status was 0 (34%) or 1 (65%), 50% with PD-L1 ≥50%, 29% with squamous and 71% with non-squamous histology, 10% had brain metastases, and 85% were former/current smokers.
The study demonstrated a statistically significant improvement in OS for PD-L1 ≥1% patients randomized to the YERVOY and nivolumab arm compared to platinum-doublet chemotherapy arm. The OS results are presented in Table 29 and Figure 5.
Table 29: Efficacy Results (PD-L1 ≥1%) - CHECKMATE-227 Part 1a a Kaplan-Meier estimate.
b Based on a stratified Cox proportional hazard model.YERVOY and Nivolumab
(n=396)Platinum-Doublet Chemotherapy
(n=397)Overall Survival
Events (%)
258 (65%)
298 (75%)
Median (months)a
(95% CI)
17.1
(15, 20.1)
14.9
(12.7, 16.7)
Hazard ratio (95% CI)b
0.79 (0.67, 0.94)
Stratified log-rank p-value
0.0066
Figure 5: Overall Survival (PD-L1 ≥1%) - CHECKMATE-227
BICR-assessed PFS showed a HR of 0.82 (95% CI: 0.69, 0.97), with a median PFS of 5.1 months (95% CI: 4.1, 6.3) in the YERVOY and nivolumab arm and 5.6 months (95% CI: 4.6, 5.8) in the platinum-doublet chemotherapy arm. The BICR-assessed confirmed ORR was 36% (95% CI: 31, 41) in the YERVOY and nivolumab arm and 30% (95% CI: 26, 35) in the platinum-doublet chemotherapy arm. Median duration of response observed in the YERVOY and nivolumab arm was 23.2 months and 6.2 months in the platinum-doublet chemotherapy arm.
First-line Treatment of Metastatic or Recurrent NSCLC: In Combination with Nivolumab and Platinum-Doublet Chemotherapy
CHECKMATE-9LA (NCT03215706) was a randomized, open-label trial in patients with metastatic or recurrent NSCLC. The trial included patients (18 years of age or older) with histologically confirmed Stage IV or recurrent NSCLC (per the 7th International Association for the Study of Lung Cancer classification [IASLC]), ECOG performance status 0 or 1, and no prior anticancer therapy (including EGFR and ALK inhibitors) for metastatic disease. Patients were enrolled regardless of their tumor PD-L1 status. Patients with known EGFR mutations or ALK translocations sensitive to available targeted inhibitor therapy, untreated brain metastases, carcinomatous meningitis, active autoimmune disease, or medical conditions requiring systemic immunosuppression were excluded from the study. Patients with stable brain metastases were eligible for enrollment.
Patients were randomized 1:1 to receive either:
- •
- YERVOY 1 mg/kg administered intravenously over 30 minutes every 6 weeks, nivolumab 360 mg administered intravenously over 30 minutes every 3 weeks, and platinum-doublet chemotherapy administered intravenously every 3 weeks for 2 cycles, or
- •
- platinum-doublet chemotherapy administered every 3 weeks for 4 cycles.
Platinum-doublet chemotherapy consisted of either carboplatin (AUC 5 or 6) and pemetrexed 500 mg/m2, or cisplatin 75 mg/m2 and pemetrexed 500 mg/m2 for non-squamous NSCLC; or carboplatin (AUC 6) and paclitaxel 200 mg/m2 for squamous NSCLC. Patients with non-squamous NSCLC in the control arm could receive optional pemetrexed maintenance therapy. Stratification factors for randomization were tumor PD-L1 expression level (≥1% versus <1% or non‑quantifiable), histology (squamous versus non-squamous), and sex (male versus female). Study treatment continued until disease progression, unacceptable toxicity, or for up to 2 years. Treatment could continue beyond disease progression if a patient was clinically stable and was considered to be deriving clinical benefit by the investigator. Patients who discontinued combination therapy because of an adverse reaction attributed to YERVOY were permitted to continue nivolumab as a single agent as part of the study. Tumor assessments were performed every 6 weeks from the first dose of study treatment for the first 12 months, then every 12 weeks until disease progression or study treatment was discontinued. The primary efficacy outcome measure was OS. Additional efficacy outcome measures included PFS, ORR, and duration of response as assessed by BICR.
A total of 719 patients were randomized to receive either YERVOY in combination with nivolumab and platinum-doublet chemotherapy (n=361) or platinum-doublet chemotherapy (n=358). The median age was 65 years (range: 26 to 86) with 51% of patients ≥65 years and 10% of patients ≥75 years. The majority of patients were White (89%) and male (70%). Baseline ECOG performance status was 0 (31%) or 1 (68%), 57% had tumors with PD-L1 expression ≥1% and 37% had tumors with PD-L1 expression that was <1%, 32% had tumors with squamous histology and 68% had tumors with non-squamous histology, 17% had CNS metastases, and 86% were former or current smokers.
The study demonstrated a statistically significant benefit in OS, PFS, and ORR. Efficacy results from the prespecified interim analysis when 351 events were observed (87% of the planned number of events for final analysis) are presented in Table 30.
Table 30: Efficacy Results - CHECKMATE-9LA a Based on a stratified Cox proportional hazard model.
b p-value is compared with the allocated alpha of 0.033 for this interim analysis.
c p-value is compared with the allocated alpha of 0.0252 for this interim analysis.
d Kaplan-Meier estimate.
e Confidence interval based on the Clopper and Pearson Method.
f p-value is compared with the allocated alpha of 0.025 for this interim analysis.YERVOY and Nivolumab and Platinum-Doublet Chemotherapy
(n=361)Platinum-Doublet Chemotherapy
(n=358)Overall Survival
Events (%)
156 (43.2)
195 (54.5)
Median (months)
(95% CI)14.1
(13.2, 16.2)10.7
(9.5, 12.5)Hazard ratio (96.71% CI)a
0.69 (0.55, 0.87)
Stratified log-rank p-valueb
0.0006
Progression-free Survival per BICR
Events (%)
232 (64.3)
249 (69.6)
Hazard ratio (97.48% CI)a
0.70 (0.57, 0.86)
Stratified log-rank p-valuec
0.0001
Median (months)d
(95% CI)6.8
(5.6, 7.7)5.0
(4.3, 5.6)Overall Response Rate per BICR (%)
38
25
(95% CI)e
(33, 43)
(21, 30)
Stratified CMH test p-valuef
0.0003
Duration of Response per BICR
Median (months)
(95% CI)d10.0
(8.2, 13.0)5.1
(4.3, 7.0)With an additional 4.6 months of follow-up the hazard ratio for overall survival was 0.66 (95% CI: 0.55, 0.80) and median survival was 15.6 months (95% CI: 13.9, 20.0) and 10.9 months (95% CI: 9.5, 12.5) for patients receiving YERVOY and nivolumab and platinum-doublet chemotherapy or platinum-doublet chemotherapy, respectively (Figure 6).
Figure 6: Overall Survival - CHECKMATE-9LA
14.7 Malignant Pleural Mesothelioma
CHECKMATE-743 (NCT02899299) was a randomized, open-label trial in patients with unresectable malignant pleural mesothelioma. The trial included patients with histologically confirmed and previously untreated malignant pleural mesothelioma with no palliative radiotherapy within 14 days of initiation of therapy. Patients with interstitial lung disease, active autoimmune disease, medical conditions requiring systemic immunosuppression, or active brain metastasis were excluded from the trial. Patients were randomized 1:1 to receive either:
- •
- YERVOY 1 mg/kg over 30 minutes by intravenous infusion every 6 weeks and nivolumab 3 mg/kg over 30 minutes by intravenous infusion every 2 weeks for up to 2 years, or
- •
- cisplatin 75 mg/m2 and pemetrexed 500 mg/m2, or carboplatin 5 AUC and pemetrexed 500 mg/m2 administered every 3 weeks for 6 cycles.
Stratification factors for randomization were tumor histology (epithelioid vs. sarcomatoid or mixed histology subtypes) and sex (male vs. female). Study treatment continued for up to 2 years, or until disease progression or unacceptable toxicity. Patients who discontinued combination therapy because of an adverse reaction attributed to YERVOY were permitted to continue nivolumab as a single agent. Treatment could continue beyond disease progression if a patient was clinically stable and was considered to be deriving clinical benefit by the investigator. Tumor assessments were performed every 6 weeks from the first dose of study treatment for the first 12 months, then every 12 weeks until disease progression or study treatment was discontinued. The primary efficacy outcome measure was OS. Additional efficacy outcome measures included PFS, ORR, and duration of response as assessed by BICR utilizing modified RECIST criteria.
A total of 605 patients were randomized to receive either YERVOY in combination with nivolumab (n=303) or chemotherapy (n=302). The median age was 69 years (range: 25 to 89), with 72% of patients ≥65 years and 26% ≥75 years; 85% were White, 11% were Asian, and 77% were male. Baseline ECOG performance status was 0 (40%) or 1 (60%), 35% had Stage III and 51% had Stage IV disease, 75% had epithelioid and 25% had non-epithelioid histology, 75% had tumors with PD-L1 expression ≥1%, and 22% had tumors with PD-L1 expression <1%.
The trial demonstrated a statistically significant improvement in OS for patients randomized to YERVOY in combination with nivolumab compared to chemotherapy. Efficacy results from the prespecified interim analysis are presented in Table 31 and Figure 7.
Table 31: Efficacy Results - CHECKMATE-743 a At the time of the interim analysis, 419 deaths (89% of the deaths needed for the final analysis) had occurred.
b Kaplan-Meier estimate.
c Stratified Cox proportional hazard model.
d p-value is compared with the allocated alpha of 0.0345 for this interim analysis.
e Based on confirmed response by BICR.YERVOY and Nivolumab
(n=303)Chemotherapy
(n=302)Overall Survivala
Events (%)
200 (66)
219 (73)
Median (months)b
(95% CI)
18.1
(16.8, 21.5)14.1
(12.5, 16.2)Hazard ratio (95% CI)c
0.74 (0.61, 0.89)
Stratified log-rank p-valued
0.002
Progression-free Survival
Events (%)
218 (72)
209 (69)
Hazard ratio (95% CI)c
1.0 (0.82, 1.21)
Median (months)b
(95% CI)
6.8
(5.6, 7.4)7.2
(6.9, 8.1)Overall Response Ratee
40%
43%
(95% CI)
(34, 45)
(37, 49)
Duration of Response
Median (months)a
(95% CI)11.0
(8.1, 16.5)6.7
(5.3, 7.1)Figure 7: Overall Survival - CHECKMATE-743
In a prespecified exploratory analysis based on histology, in the subgroup of patients with epithelioid histology, the hazard ratio (HR) for OS was 0.85 (95% CI: 0.68, 1.06), with median OS of 18.7 months in the YERVOY and nivolumab arm and 16.2 months in the chemotherapy arm. In the subgroup of patients with non-epithelioid histology, the HR for OS was 0.46 (95% CI: 0.31, 0.70), with median OS of 16.9 months in the YERVOY and nivolumab arm and 8.8 months in the chemotherapy arm.
14.8 Esophageal Cancer
CHECKMATE-648 (NCT03143153) was a randomized, active-controlled, open-label trial in patients with previously untreated unresectable advanced, recurrent or metastatic ESCC (squamous or adenosquamous histology). The trial enrolled patients whose tumor was evaluable for tumor cell (TC) PD-L1 expression [also called PD-L1 tumor proportion score (TPS)], which was evaluated using the PD-L1 IHC 28-8 pharmDx assay at a central laboratory. Patients were not amenable to chemoradiation or surgery with curative intent. A retrospective scoring of a patient’s tumor PD-L1 status using Combined Positive Score (CPS), was also conducted using the PD-L1-stained tumor specimens used for randomization. Prior treatment with curative intent was allowed if completed more than six months prior to trial enrollment. The trial excluded patients with brain metastasis that were symptomatic, had active autoimmune disease, used systemic corticosteroids or immunosuppressants, or patients at high risk of bleeding or fistula due to apparent invasion of tumor to organs adjacent to the esophageal tumor. Patients were randomized to receive one of the following treatments:
- •
- YERVOY 1 mg/kg every 6 weeks in combination with nivolumab 3 mg/kg every 2 weeks.
- •
- Fluorouracil 800 mg/m2/day intravenously on days 1 through 5 (for 5 days), and cisplatin 80 mg/m2 intravenously on day 1 (of a 4-week cycle).
Patients were treated until disease progression, unacceptable toxicity, or up to 2 years. Patients who discontinued combination therapy because of an adverse reaction attributed to YERVOY were permitted to continue nivolumab as a single agent.
Randomization was stratified by TC PD-L1 expression (≥1% vs. <1% or indeterminate), region (East Asia vs. Rest of Asia vs. Rest of World), ECOG performance status (0 vs. 1), and number of organs with metastases (≤1 vs. ≥2). The major efficacy outcome measures were OS and BICR-assessed PFS in patients with TC PD-L1 expression ≥ 1%. Additional efficacy measures included OS in all randomized patients, BICR-assessed PFS in all randomized patients, and ORR assessed by BICR in TC PD-L1 expression ≥1% and in all randomized patients. The tumor assessments per RECIST v1.1 were conducted every 6 weeks up to and including week 48, then every 12 weeks thereafter.
A total of 649 patients were randomized in Arms A and C of CHECKMATE-648 study among whom 644 and 601 patients had quantifiable TC PD-L1 expression and CPS at baseline, respectively. The trial population characteristics were: median age 64 years (range: 26 to 81), 46% were ≥65 years of age, 84% were male, 71% were Asian, 25% were White, and 1.5% were Black. Patients had histological confirmation of squamous cell carcinoma (99%) or adenosquamous cell carcinoma (1.4%) in the esophagus. Baseline ECOG performance status was 0 (46%) or 1 (54%).
Efficacy results are shown in Table 32 and Figure 8.
Table 32: Efficacy Results - Arms A and C of CHECKMATE-648 a Assessed by BICR.
b Based on stratified Cox proportional hazard model. Hazard ratios are reported for each OPDIVO containing arm compared to chemotherapy within each analysis population.
c Based on a stratified 2-sided log-rank test.
S1, S2 Significant p-value compared to stopping boundary of 0.018, and 0.014 respectively.
NS: Not Statistically significant, NT: Not evaluated for statistical significance as per pre-specified hierarchical testing procedureYERVOY and Nivolumab
(n=325)Cisplatin and Fluorouracil
(n=324)YERVOY and Nivolumab
(n=158)Cisplatin and Fluorouracil
(n=157)All Patients
TC PD-L1 expression ≥ 1%
Overall Survival
Deaths (%)
216 (66)
232 (72)
106 (67)
121 (77)
Median (months)
(95% CI)
12.8
(11.3, 15.5)
10.7
(9.4, 11.9)
13.7
(11.2, 17.0)
9.1
(7.7, 10)
Hazard ratio (95% CI)b
0.78
(0.65, 0.95)
-
0.64
(0.49, 0.84)
-
p-valuec
0.0110 S1
-
0.0010S2
-
Progression-free Survivala
Disease progression or death (%)
258 (79)
210 (65)
123 (78)
100 (64)
Median (months)
(95% CI)
2.9 (2.7, 4.2)
5.6
(4.3, 5.9)
4.0
(2.4, 4.9)
4.4
(2.9, 5.8)
Hazard ratio (95% CI)b
1.26
(1.04, 1.52)
-
1.02
(0.78, 1.34)
-
p-valuec
NT
-
NS
-
Overall Response Rate, n (%)a, NT
90 (27.7)
87 (26.9)
56 (35.4)
31 (19.7)
(95% CI)
(22.9, 32.9)
(22.1, 32.0)
(28.0, 43.4)
(13.8, 26.8)
Complete response (%)
36 (11.1)
20 (6.2)
28 (17.7)
8 (5.1)
Partial response (%)
54 (16.6)
67 (20.7)
28 (17.7)
23 (14.6)
Median
(95% CI)
11.1
(8.3, 14.0)
7.1
(5.7, 8.2)
11.8
(7.1, 27.4)
5.7
(4.4, 8.7)
Range
1.4+, 34.5+
1.4+, 31.8+
1.4+, 34.5+
1.4+, 31.8+
Figure 8: Overall Survival (All Randomized) - CHECKMATE-648
(A) OS in All Randomized Patients
(B) OS in TC PD-L1 ≥1%
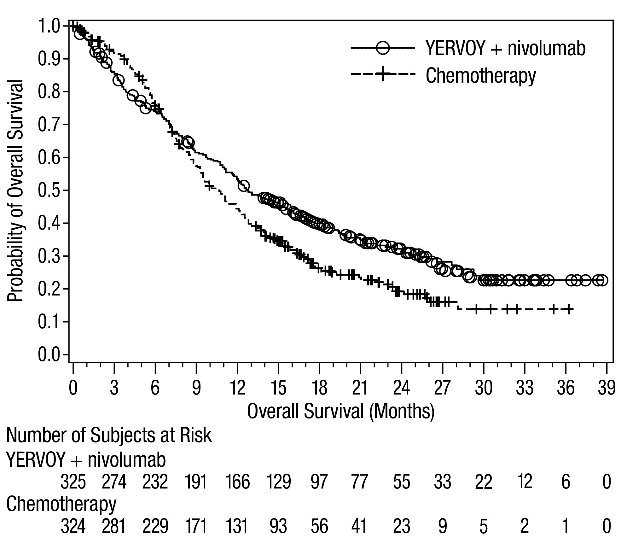
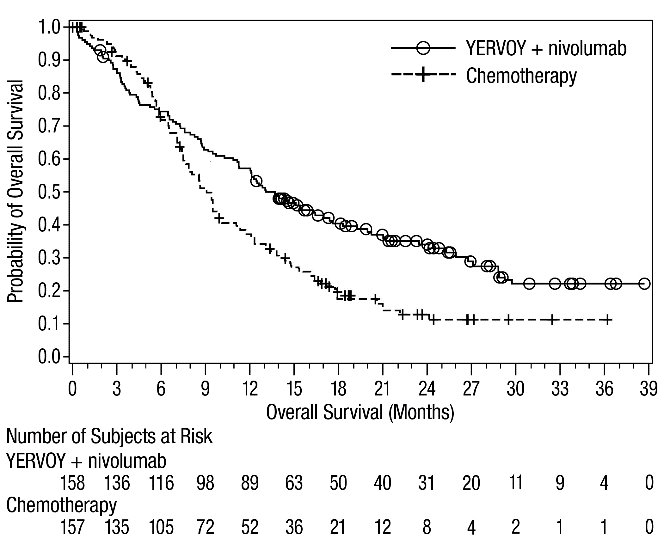
Figure 9: Progression Survival – CHECKMATE-648
(A) PFS in All Randomized Patients
(B) PFS in TC PD-L1 ≥1%
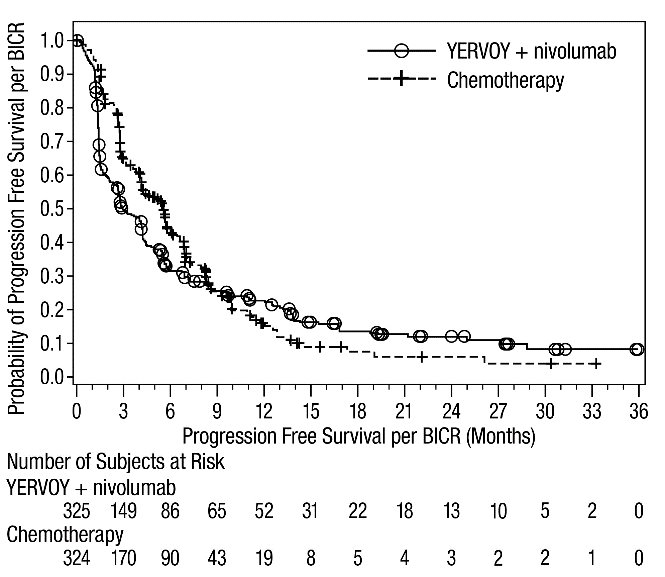
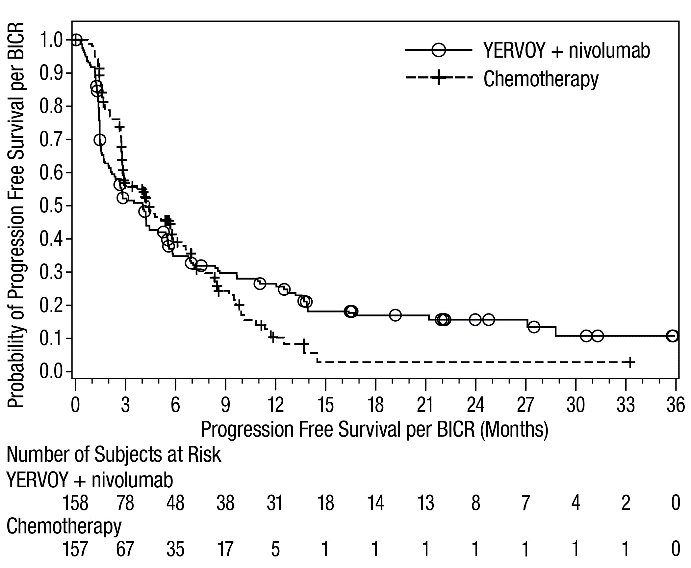
An exploratory subgroup analysis of patients with TC PD-L1 expression<1% (n=329) was conducted. The unstratified OS HR for YERVOY with nivolumab (n=164) vs. chemotherapy (n=165) was 0.97 (95% CI: 0.74, 1.26) with median OS of 12 months (95% CI: 10.1, 16.0) on the YERVOY with nivolumab arm and 12.2 months (95% CI: 10.7, 14) on the chemotherapy arm.
Exploratory subgroup analyses were also conducted by PD-L1 status per CPS (≥1 and <1) for YERVOY with nivolumab arm compared to chemotherapy. Among the 601 patients with quantifiable PD-L1 CPS at baseline, 280 in the chemotherapy arm, and 266 in the YERVOY with nivolumab arm had PD-L1 CPS≥1. A total of 24 patients in the chemotherapy arm and 31 patients in the YERVOY with nivolumab arm had PD-L1 CPS<1. The unstratified OS HR was 0.76 (95% CI: 0.62, 0.93) for PD-L1 CPS≥1 subgroup and 1.0 (95% CI: 0.52, 1.94) for PD-L1 CPS<1 subgroup.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
YERVOY (ipilimumab) injection is a sterile, preservative-free, clear to slightly opalescent, colorless to pale-yellow solution. YERVOY is available as follows:
Carton Contents
NDC
One 50 mg/10 mL (5 mg/mL), single-dose vial
NDC 0003-2327-11
One 200 mg/40 mL (5 mg/mL), single-dose vial
NDC 0003-2328-22
Store YERVOY under refrigeration at 2°C to 8°C (36°F to 46°F). Protect YERVOY from light by storing in the original carton until time of use. Do not freeze or shake.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Immune-Mediated Adverse Reactions
Advise patients that YERVOY can cause immune-mediated adverse reactions including the following [see Warnings and Precautions (5.1)]:
- •
- Immune-Mediated Diarrhea or Colitis: Advise patients to contact their healthcare provider immediately for signs or symptoms of diarrhea or colitis.
- •
- Immune-Mediated Hepatitis: Advise patients to contact their healthcare provider immediately for signs or symptoms of hepatitis.
- •
- Immune-Mediated Dermatologic Adverse Reactions: Advise patients to contact their healthcare provider immediately if they develop a new rash.
- •
- Immune-Mediated Endocrinopathies: Advise patients to contact their healthcare provider immediately for signs or symptoms of hypophysitis, adrenal insufficiency, hypothyroidism, hyperthyroidism, and diabetes mellitus
- •
- Immune-Mediated Pneumonitis: Advise patients to contact their healthcare provider immediately for any new or worsening symptoms of pneumonitis.
- •
- Immune-Mediated Nephritis with Renal Dysfunction: Advise patients to contact their healthcare provider immediately for signs or symptoms of nephritis.
Infusion-Related Reactions
- •
- Advise patients who are receiving YERVOY of the potential risk of an infusion-related reaction [see Warnings and Precautions (5.2)].
Embryo-Fetal Toxicity
- •
- Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.4), Use in Specific Populations (8.3)].
- •
- Advise females of reproductive potential to use effective contraception during treatment with YERVOY and for 3 months after the last dose [see Use in Specific Populations (8.3)].
- •
- Advise patients who may have been exposed to YERVOY during pregnancy to contact Bristol-Myers Squibb at 1-844-593-7869 [see Use in Specific Populations (8.1)].
Lactation
- •
- Advise women not to breastfeed during treatment with YERVOY and for 3 months after the last dose [see Use in Specific Populations (8.2)].
-
MEDICATION GUIDE
MEDICATION GUIDE
YERVOY® (yur-voi)
(ipilimumab)
injectionRead this Medication Guide before you start receiving YERVOY and before each infusion. There may be new information. If your healthcare provider prescribes YERVOY in combination with nivolumab (OPDIVO®), also read the Medication Guide that comes with nivolumab. This Medication Guide does not take the place of talking with your healthcare provider about your medical condition or your treatment.
What is the most important information I should know about YERVOY?
YERVOY is a medicine that may treat certain cancers by working with your immune system. YERVOY can cause your immune system to attack normal organs and tissues in any area of your body and can affect the way they work. These problems can sometimes become severe or life-threatening and can lead to death. You may have more than one of these problems at the same time. These problems may happen anytime during treatment or even after your treatment has ended. Some of these problems may happen more often when YERVOY is used in combination with nivolumab.
Call or see your healthcare provider right away if you develop any new or worse signs or symptoms, including:
Intestinal problems.
- •
- diarrhea (loose stools) or more frequent bowel movements than usual
- •
- stools that are black, tarry, sticky, or have blood or mucus
- •
- severe stomach-area (abdominal) pain or tenderness
Liver problems.
- •
- yellowing of your skin or the whites of your eyes
- •
- severe nausea or vomiting
- •
- pain on the right side of your stomach-area (abdomen)
- •
- dark urine (tea colored)
- •
- bleeding or bruising more easily than normal
Skin problems.
- •
- rash
- •
- itching
- •
- skin blistering or peeling
- •
- painful sores in mouth or nose, throat, or genital area
Hormone gland problems.
- •
- headache that will not go away or unusual headaches
- •
- eye sensitivity to light
- •
- eye problems
- •
- rapid heartbeat
- •
- increased sweating
- •
- extreme tiredness
- •
- weight gain or weight loss
- •
- feeling more hungry or thirsty than usual
- •
- urinating more often than usual
- •
- hair loss
- •
- feeling cold
- •
- constipation
- •
- your voice gets deeper
- •
- dizziness or fainting
- •
- changes in mood or behavior, such as decreased sex drive, irritability, or forgetfulness
Lung problems.
- •
- new or worsening cough
- •
- shortness of breath
- •
- chest pain
Kidney problems.
- •
- decrease in your amount of urine
- •
- blood in your urine
- •
- swelling of your ankles
- •
- loss of appetite
Eye problems.
- •
- blurry vision, double vision, or other vision problems
- •
- eye pain or redness
Problems can also happen in other organs and tissues. These are not all of the signs and symptoms of immune system problems that can happen with YERVOY. Call or see your healthcare provider right away for any new or worsening signs or symptoms.
- •
- Chest pain, irregular heartbeat, shortness of breath or swelling of ankles
- •
- Confusion, sleepiness, memory problems, changes in mood or behavior, stiff neck, balance problems, tingling or numbness of the arms or legs
- •
- Double vision, blurry vision, sensitivity to light, eye pain, changes in eyesight
- •
- Persistent or severe muscle pain or weakness, muscle cramps
- •
- Low red blood cells, bruising
Getting medical treatment right away may help keep these problems from becoming more serious. Your healthcare provider will check you for these problems during your treatment with YERVOY. Your healthcare provider may treat you with corticosteroid or hormone replacement medicines. Your healthcare provider may also need to delay or completely stop treatment with YERVOY if you have severe side effects.
What is YERVOY?
YERVOY is a prescription medicine used:
- •
-
to treat a kind of skin cancer called melanoma.
- o
- YERVOY may be used alone or in combination with nivolumab in adults and children 12 years of age and older when melanoma has spread or cannot be removed by surgery.
- o
- YERVOY may be used alone in adults to help prevent melanoma from coming back after it and lymph nodes that contain cancer have been removed by surgery.
- •
- in adults with kidney cancer (renal cell carcinoma). YERVOY may be used in combination with nivolumab in certain people when their cancer has spread.
- •
-
in adults and children 12 years of age and older, with a type of colon or rectal cancer (colorectal cancer).
- o
- YERVOY in combination with nivolumab may be used when your colon or rectal cancer:
- ▪
- has spread to other parts of the body (metastatic),
- ▪
- is microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR), and
- ▪
- you have tried treatment with a fluoropyrimidine, oxaliplatin, and irinotecan, and it did not work or is no longer working.
- •
-
in adults with liver cancer (hepatocellular carcinoma).
- o
- YERVOY may be used in combination with nivolumab if you have previously received treatment with sorafenib.
- •
-
in adults with a type of lung cancer called non-small cell lung cancer (NSCLC).
- o
- YERVOY may be used in combination with nivolumab as your first treatment for NSCLC:
- ▪
- when your lung cancer has spread to other parts of your body (metastatic), and
- ▪
- your tumors are positive for PD-L1, but do not have an abnormal EGFR or ALK gene.
- o
- YERVOY may be used in combination with nivolumab and 2 cycles of chemotherapy that contains platinum and another chemotherapy medicine, as the first treatment of your NSCLC when your lung cancer:
- ▪
- has spread or grown, or comes back, and
- ▪
- your tumor does not have an abnormal EGFR or ALK gene.
- •
-
in adults with a type of cancer that affects the lining of the lungs and chest wall called malignant pleural mesothelioma.
- o
- YERVOY may be used in combination with nivolumab as your first treatment for malignant pleural mesothelioma that cannot be removed by surgery.
- •
-
in adults with cancer of the tube that connects your throat to your stomach (esophageal cancer).
- o
- YERVOY may be used in combination with nivolumab when your esophageal cancer:
- ▪
- is a type called squamous cell carcinoma, and
- ▪
- cannot be removed with surgery (advanced), or has spread to the other parts of the body (metastatic), and
- ▪
- you have not already had treatment for your advanced or metastatic esophageal cancer.
It is not known if YERVOY is safe and effective in children younger than 12 years of age with melanoma that has spread or cannot be removed by surgery, or with MSI-H or dMMR metastatic colorectal cancer.
It is not known if YERVOY is safe and effective in children for the treatment of any other cancers.
Before you receive YERVOY, tell your healthcare provider about all your medical conditions, including if you:
- •
- have immune system problems such as ulcerative colitis, Crohn’s disease, or lupus
- •
- have received an organ transplant
- •
- have received or plan to receive a stem cell transplant that uses donor stem cells (allogeneic)
- •
- have a condition that affects your nervous system, such as myasthenia gravis or Guillain-Barré syndrome
- •
- are pregnant or plan to become pregnant. YERVOY can harm your unborn baby.
-
Females who are able to become pregnant:
- o
- Your healthcare provider will give you a pregnancy test before you start treatment with YERVOY.
- o
- You should use an effective method of birth control during treatment and for 3 months after your last dose of YERVOY. Talk to your healthcare provider about birth control methods that you can use during this time.
- o
- Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with YERVOY. You or your healthcare provider should contact Bristol-Myers Squibb at 1-844-593-7869 as soon as you become aware of a pregnancy.
- •
- are breastfeeding or plan to breastfeed. It is not known if YERVOY passes into your breast milk. Do not breastfeed during treatment and for 3 months after your last dose of YERVOY.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How will I receive YERVOY?
- •
- Your healthcare provider will give you YERVOY into your vein through an intravenous (IV) line.
- •
- YERVOY is usually given over 30 minutes.
- •
- If you are receiving YERVOY as treatment for melanoma that has been removed by surgery to help prevent it from coming back, YERVOY will be given over 90 minutes.
- •
- In combination with nivolumab, YERVOY is usually given every 3 weeks for 4 doses. After that, nivolumab alone is usually given every 2 or 4 weeks.
- •
- For NSCLC that has spread to other parts of your body, YERVOY is given every 6 weeks and nivolumab is given every 3 weeks for up to 2 years. Your healthcare provider will determine if you will also need to receive chemotherapy every 3 weeks for 2 cycles.
- •
- For malignant pleural mesothelioma that cannot be removed by surgery, YERVOY is given every 6 weeks and nivolumab is given every 3 weeks for up to 2 years.
- •
- When YERVOY is used in combination with nivolumab for esophageal squamous cell carcinoma (ESCC), YERVOY is given every 6 weeks and nivolumab is given every 2 or 3 weeks for up to 2 years.
- •
- Your healthcare provider will decide how many treatments you will need.
- •
- Your healthcare provider will do blood tests before starting and during treatment with YERVOY.
- •
- If you miss any appointments, call your healthcare provider as soon as possible to reschedule your appointment.
What are the possible side effects of YERVOY?
YERVOY can cause serious side effects, including:
- •
- See “What is the most important information I should know about YERVOY?”
- •
- Severe infusion-related reactions. Tell your healthcare provider or nurse right away if you get these symptoms during an infusion of YERVOY:
- o
- chills or shaking
- o
- itching or rash
- o
- flushing
- o
- shortness of breath or wheezing
- o
- dizziness
- o
- feel like passing out
- o
- fever
- o
- back or neck pain
- •
- Complications, including graft-versus-host disease (GVHD), in people who have received a bone marrow (stem cell) transplant that uses donor stem cells (allogeneic). These complications can be severe and can lead to death. These complications may happen if you underwent transplantation either before or after being treated with YERVOY. Your healthcare provider will monitor you for these complications.
The most common side effects of YERVOY when used alone include:
- •
- feeling tired
- •
- diarrhea
- •
- nausea
- •
- itching
- •
- rash
- •
- vomiting
- •
- headache
- •
- weight loss
- •
- fever
- •
- decreased appetite
- •
- difficulty falling or staying asleep
The most common side effects of YERVOY when used in combination with nivolumab include:
- •
- feeling tired
- •
- diarrhea
- •
- rash
- •
- itching
- •
- nausea
- •
- pain in muscles, bones, and joints
- •
- fever
- •
- cough
- •
- decreased appetite
- •
- vomiting
- •
- stomach-area (abdominal) pain
- •
- shortness of breath
- •
- upper respiratory tract infection
- •
- headache
- •
- low thyroid hormone levels (hypothyroidism)
- •
- constipation
- •
- decreased weight
- •
- dizziness
The most common side effects of YERVOY when used in combination with nivolumab and chemotherapy include:
- •
- feeling tired
- •
- pain in muscles, bones, and joints
- •
- nausea
- •
- diarrhea
- •
- rash
- •
- decreased appetite
- •
- constipation
- •
- itching
These are not all of the possible side effects of YERVOY.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of YERVOY.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. If you would like more information about YERVOY, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about YERVOY that is written for health professionals.
What are the ingredients of YERVOY?
Active ingredient: ipilimumab
Inactive ingredients: diethylene triamine pentaacetic acid (DTPA), mannitol, polysorbate 80 (vegetable origin), sodium chloride, tris hydrochloride, and Water for Injection
Manufactured by: Bristol-Myers Squibb Company, Princeton, NJ 08543 USA
U.S. License No. 1713YERVOY® and OPDIVO® are trademarks of Bristol-Myers Squibb Company. All other trademarks are the property of their respective owners.
For more information, call 1-800-321-1335
This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: February 2023
-
YERVOY 50 mg/10 mL Representative Packaging
See How Supplied section for a complete list of available packages of YERVOY.
NDC 0003-2327-11
Rx only
YERVOY®
(ipilimumab)
Injection
50 mg/10 mL
(5 mg/mL)
For Intravenous Infusion Only
Single-use vial; Discard unused portion
DISPENSE ENCLOSED MEDICATION GUIDE TO EACH PATIENT
Bristol-Myers Squibb - YERVOY 200 mg/40 mL Representative Packaging
-
INGREDIENTS AND APPEARANCE
YERVOY
ipilimumab injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0003-2327 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IPILIMUMAB (UNII: 6T8C155666) (IPILIMUMAB - UNII:6T8C155666) IPILIMUMAB 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength TROMETHAMINE HYDROCHLORIDE (UNII: 383V75M34E) 3.15 mg in 1 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) 5.85 mg in 1 mL MANNITOL (UNII: 3OWL53L36A) 10 mg in 1 mL PENTETIC ACID (UNII: 7A314HQM0I) 0.04 mg in 1 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) 0.1 mg in 1 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0003-2327-11 1 in 1 CARTON 03/25/2011 1 10 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125377 03/25/2011 YERVOY
ipilimumab injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0003-2328 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IPILIMUMAB (UNII: 6T8C155666) (IPILIMUMAB - UNII:6T8C155666) IPILIMUMAB 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength TROMETHAMINE HYDROCHLORIDE (UNII: 383V75M34E) 3.15 mg in 1 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) 5.85 mg in 1 mL MANNITOL (UNII: 3OWL53L36A) 10 mg in 1 mL PENTETIC ACID (UNII: 7A314HQM0I) 0.04 mg in 1 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) 0.1 mg in 1 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0003-2328-22 1 in 1 CARTON 03/25/2011 1 40 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125377 03/25/2011 Labeler - E.R. Squibb & Sons, L.L.C. (011550092)