DOXORUBICIN HYDROCHLORIDE- doxorubicin hydrochloride injection, powder, lyophilized, for solution
Hospira, Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
DOXORUBICIN HYDROCHLORIDE
Health Care Provider Letter
Important Drug Information
|
Subject: |
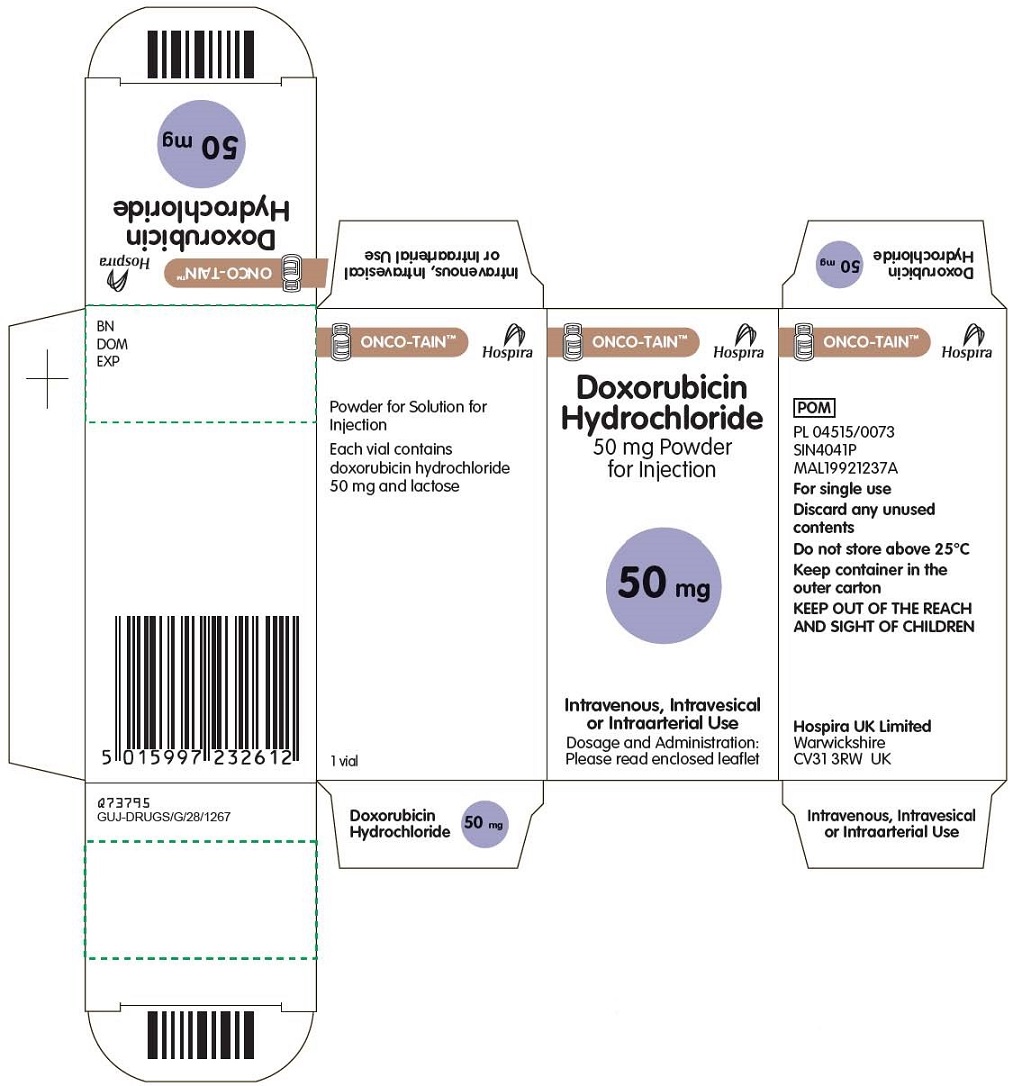
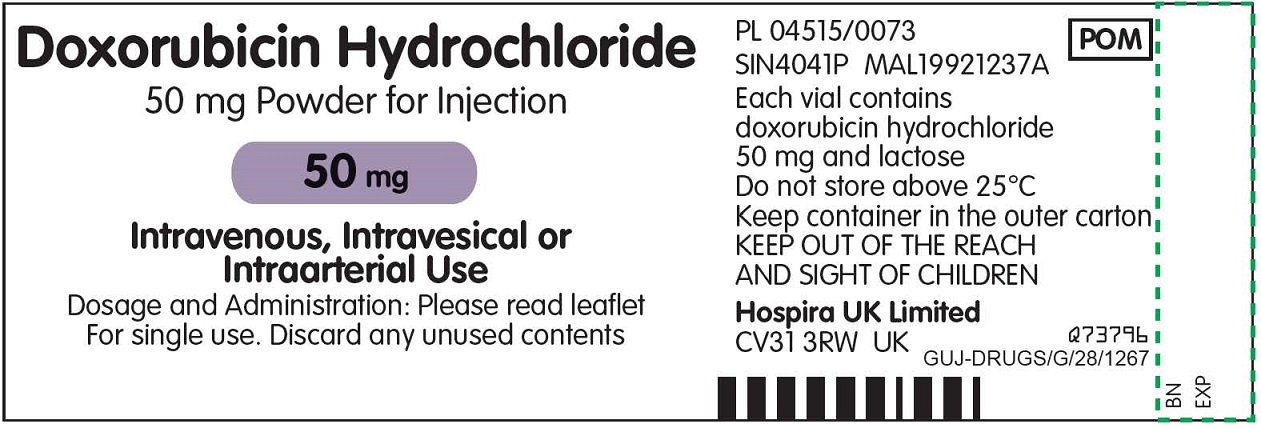
Temporary importation of Doxorubicin Hydrochloride 50 mg Powder for Injection (50 mg/vial) to address drug shortage issue |
April 18, 2016
Dear Health Care Provider,
Due to the current critical shortage of Doxorubicin Hydrochloride for Injection, USP in the United States (U.S.) market, Hospira, Inc., a Pfizer company (Hospira), is coordinating with the U.S. Food and Drug Administration (FDA) to increase the availability of the drug. Hospira has initiated temporary importation of non-U.S. approved product, Doxorubicin Hydrochloride 50 mg Powder for Injection from Hospira Limited, United Kingdom (Hospira Limited): Lot DO21502A, expiration 12/2017.
The Doxorubicin Hydrochloride 50 mg Powder for Injection from Hospira Limited is manufactured by Zydus Hospira Oncology Private Limited (ZHOPL) in Ahmedabad, India, at an FDA-inspected facility that is in compliance with current good manufacturing practice requirements.
At this time, no other entity except Hospira Limited or their distributor Hospira is authorized by the FDA to import or distribute Doxorubicin Hydrochloride 50 mg Powder for Injection in the U.S. FDA has not approved Hospira Limited’s Doxorubicin Hydrochloride 50 mg Powder for Injection (50 mg/vial) product in the U.S.
Effective immediately, and during this temporary period, Hospira will offer the following presentation of Doxorubicin Hydrochloride for Injection:
|
Product |
Strength |
Size |
Marketing Authorization # |
|
Doxorubicin Hydrochloride 50 mg Powder for Injection |
50 mg/vial |
Box of 1 vial |
PL 4515/0073 (United Kingdom) |
The barcode used for Hospira Limited’s non-U.S. approved Doxorubicin Hydrochloride 50 mg Powder for Injection is an international pharmaceutical manufacturing code and may not be appropriately recognized by scanning systems used in the U.S. Institutions should confirm the barcode systems do not provide incorrect information when the product is scanned. Alternative procedures should be followed to assure that the correct drug product is being used and administered to individual patients.
It is important to note that there are substantive differences in the format and content of the labeling between the U.S.-approved Doxorubicin Hydrochloride for Injection, USP and the Hospira Limited’s Doxorubicin Hydrochloride 50 mg Powder for Injection. The product comparison tables attached highlight the differences between the reference listed drug (RLD) Doxorubicin Hydrochloride for Injection, USP and Hospira Limited’s non-U.S. approved Doxorubicin Hydrochloride 50 mg Powder for Injection.
This letter and the attachments are not intended as a complete description of the benefits and risks related to the use of Doxorubicin Hydrochloride 50 mg Powder for Injection. Please refer to the package insert for the FDA-approved Doxorubicin Hydrochloride for Injection, USP for full prescribing information.
To place an order, or if you have any questions, customers can contact Hospira directly by calling Customer Care at 1-877-946-7747 (Monday – Friday, 7AM-6PM CDT).
Hospira will make reasonable attempts to fill your orders. Hospira will be closely monitoring the distribution of Doxorubicin Hydrochloride 50 mg Powder for Injection to help manage supply.
For clinical inquiries, please contact Hospira Medical Communications at 1-800-615-0187 (available 24 hours a day/7 days per week), or email at medcom@hospira.com.
To report adverse events or quality problems associated with the use of this product, please call Hospira Global Complaint Management at 1-800-441-4100 (Monday – Friday, 8AM-5PM CDT), or email at ProductComplaintsPP@hospira.com.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- •
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm.
- •
- Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178 (1-800-332-0178).
For full U.S.-approved Prescribing Information for Doxorubicin Hydrochloride for Injection, including the BOXED WARNING, please visit http://www.accessdata.fda.gov/spl/data/bb405a38-3e4e-49af-b30d-fb728f209022/bb405a38-3e4e-49af-b30d-fb728f209022.xml
For Full Prescribing information for Hospira Limited’s product, please visit http://www.pfizerinjectables.com/sites/default/files/doxorubicinSmPC-HospiraUK
Sincerely,
Janet Stevens
Vice President, Quality Operations
Hospira, Inc., a Pfizer Company
| DOXORUBICIN HYDROCHLORIDE
doxorubicin hydrochloride injection, powder, lyophilized, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Hospira, Inc. (141588017) |