Label: KIT FOR THE PREPARATION OF TECHNETIUM TC99M PYROPHOSPHATE- technetium tc99m pyrophosphate injection
- NDC Code(s): 45567-0060-1, 45567-0060-2
- Packager: Sun Pharmaceutical Industries, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated August 20, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Kit for the Preparation of Technetium Tc 99m Pyrophosphate Injection is a multidose reaction vial which contains the sterile, non-pyrogenic, non-radioactive ingredients necessary to produce Technetium Tc 99m Pyrophosphate Injection for diagnostic use by intravenous injection.
Each 10 mL vial contains 12.0 mg of sodium pyrophosphate, 2.8 mg minimum stannous tin as stannous chloride dihydrate and 4.9 mg maximum total tin as stannous chloride dihydrate; pH is adjusted to 5.3-5.7 with hydrochloric acid prior to lyophilization. No bacteriostatic preservative is present. Sealed under nitrogen.
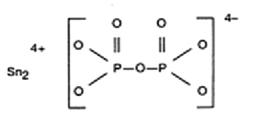
The chemical names are: (1) Diphosphoric acid, Ditin (2+) salt; (2) Ditin (2+) pyrophosphate (4-). The structural formula is:
When a solution of sterile, non-pyrogenic, oxidant-free isotonic Sodium Pertechnetate Tc 99m Injection U.S.P. is added to the vial, Technetium Tc 99m Pyrophosphate Injection is formed for intravenous injection.
When a solution of sterile, non-pyrogenic, isotonic saline is added to the vial, it forms
a blood pool imaging agent when Sodium Pertechnetate Tc 99m Injection is injected intravenously 30 minutes after the intravenous administration of the non-radioactive reconstituted Kit for the Preparation of Technetium Tc 99m Pyrophosphate Injection. The precise structure of Technetium Tc 99m Pyrophosphate Injection is not known at this time.Physical Characteristics
Technetium Tc 99m decays by isomeric transition with a physical half-life of 6.02 hours.¹ The principal photon that is useful for detection and imaging studies is listed in Table 1.
TABLE 1: Principal Radiation Emission Data Radiation
Mean Percent Per Disintegration
Mean Energy (keV)
Gamma-2
89.07
140.5
¹Kocher DC: Radioactive decay data tables. DOE/TIC-11026: 108, 1981
External Radiation
The specific gamma ray constant for Tc 99m is 0.78 R/hr-millicurie at 1 cm. The first half-value layer is 0.017 cm of lead (Pb). A range of values for the relative attenuation of the radiation emitted by this radionuclide that results from interposition of various thicknesses of Pb is shown in Table 2. For example, the use of a 0.25 cm thickness of Pb will attenuate the radiation emitted by a factor of about 1,000.
TABLE 2: Radiation Attenuation by Lead Shielding Shield Thickness
(Pb) cmCoefficient of
Attenuation0.017
0.5
0.08
10-1
0.16
10-2
0.25
10-3
0.33
10-4
To correct for physical decay of this radionuclide, the fractions that remain at selected intervals after the time of calibration are shown in Table 3.
TABLE 3: Physical Decay Chart: Tc 99m, half-life 6.02 hours Hours
Fraction
RemainingHours
Fraction
Remaining0*
1.000
7
0.447
1
0.891
8
0.398
2
0.794
9
0.355
3
0.708
10
0.316
4
0.631
11
0.282
5
0.562
12
0.251
6
0.501
* Calibration time
-
CLINICAL PHARMACOLOGY
When injected intravenously, Technetium Tc 99m Pyrophosphate Injection has a specific affinity for areas of osteogenesis. It is also concentrated in the injured myocardium, primarily in areas of irreversibly damaged myocardial cells.
One to two hours after intravenous injection of Technetium Tc 99m Pyrophosphate Injection, an estimated 40 to 50 percent of the injected dose has been taken up by the skeleton, and approximately 0.01 to 0.02 percent per gram of acutely infarcted myocardium. Within a period of one hour, 10 to 11 percent remains in the vascular system, declining to approximately 2 to 3 percent twenty-four hours post injection.
The average urinary excretion was observed to be about 40 percent of the administered dose after 24 hours.The non-radioactive reconstituted Kit for the Preparation of Technetium Tc 99m Pyrophosphate Injection also has an affinity for red blood cells. When administered 30 minutes prior to the intravenous administration of Sodium Pertechnetate Tc 99m Injection, approximately 76 percent of the injected activity remains in the blood pool providing excellent images of the cardiac chambers.
-
INDICATIONS AND USAGE
Technetium Tc 99m Pyrophosphate Injection is a bone imaging agent used to demonstrate areas of altered osteogenesis, and a cardiac imaging agent used as an adjunct in the diagnosis of acute myocardial infarction.
Kit for the Preparation of Technetium Tc 99m Pyrophosphate Injection is a blood pool imaging agent which may be used for gated blood pool imaging and for the detection of sites of gastrointestinal bleeding. When reconstituted with sterile non-pyrogenic isotonic saline and administered intravenously 30 minutes prior to the intravenous administration of Sodium Pertechnetate Tc 99m Injection, approximately 76% of the injected radioactivity remains in the blood pool.
- CONTRAINDICATIONS
-
WARNINGS
As an adjunct in the diagnosis of confirmed myocardial infarction (ECG and serum enzymes positive), the incidence of false negative images has been found to be 6 percent. False negative images can also occur if made prior to 24 hours in the evolutionary phase of the infarct or after 6 days in the resolution phase. In a limited study involving 22 patients in whom the ECG was positive and serum enzymes questionable or negative, but in whom the final diagnosis of acute myocardial infarction was made, the incidence of false negative images was 23 percent. The incidence of false positive images has been found to be 7 to 9 percent. False positive images have also been reported following coronary by-pass graft surgery, in unstable angina pectoris, old myocardial infarcts and in cardiac contusions.
Preliminary reports indicate impairment of brain scans using Sodium Pertechnetate Tc99m Injection which have been preceded by a bone scan using an agent containing stannous ions. The impairment may result in false positive or false negative brain scans. It is recommended, where feasible, that brain scans precede bone imaging procedures. Alternately, a brain imaging agent such as Technetium Tc 99m Pentetate Injection may be employed.
-
PRECAUTIONS
General
The lyophilized contents of the Kit for the Preparation of Technetium Tc 99m Pyrophosphate Injection reaction vial are to be administered to the patient only as an intravenous solution (see Procedures for Reconstitution). Any Sodium Pertechnetate Tc 99m solution which contains an oxidizing agent is not suitable for use with Kit for the Preparation of Technetium Tc 99m Pyrophosphate Injection. When reconstituted with Sodium Pertechnetate Tc 99m, Technetium Tc 99m Pyrophosphate Injection must be used within 6 hours. Kit for the Preparation of Technetium Tc 99m Pyrophosphate Injection may also be reconstituted with sterile, non-pyrogenic isotonic saline containing no preservatives and injected intravenously prior to the administration of Sodium Pertechnetate Tc 99m Injection.
Kit for the Preparation of Technetium Tc 99m Pyrophosphate Injection contains no preservatives.
Vials are sealed under nitrogen: air or oxygen is harmful to the contents of the vials and the vials should not be vented.
The components of the Kit for the Preparation of Technetium Tc 99m Pyrophosphate Injection are supplied sterile and non-pyrogenic. Aseptic procedures normally employed in making additions and withdrawals for sterile, non-pyrogenic containers should be used during addition of the Sodium Pertechnetate Tc 99m Injection and the withdrawal of doses for patient administration.
Shielding should be utilized when preparing Technetium Tc 99m Pyrophosphate Injection.
Technetium Tc 99m Pyrophosphate Injection as well as other radioactive drugs must be handled with care, and appropriate safety measures should be used to minimize radiation exposure to the patients and clinical personnel consistent with proper patient management.
The solution should not be used if cloudy, discolored, or found to contain particulate matter.
Radiopharmaceuticals should be used only by physicians who are qualified by training and experience in the safe use and handling of radionuclides, and whose experience and training have been approved by the appropriate government agency authorized to license the use of radionuclides.
No special handling is required for the non-radioactive drug product.
Bone Imaging
Both prior to and following Technetium Tc 99m Pyrophosphate Injection administration, if not contraindicated for the patient’s cardiac condition, patients should be encouraged to drink fluids. Patients should void as often as possible after the Technetium Tc 99m Pyrophosphate Injection to minimize background interference and unnecessary radiation exposure from accumulation in the bladder.
Cardiac Imaging
Patient’s cardiac condition should be stable before beginning the cardiac imaging procedure.
Interference from chest wall lesions such as breast tumors and healing rib fractures can be minimized by employing the three recommended projections. (See DOSAGE AND ADMINISTRATION). False-positive and false-negative myocardial scans may occur; therefore, the diagnosis of acute myocardial infarction depends on the overall assessment of laboratory and clinical findings.
Blood Pool Imaging
The non-radioactive reconstituted agent should be injected by direct venipuncture. Heparinized catheter systems should be avoided, as interference with red blood cell tagging will result. Cardiac pool imaging should be initiated 15 to 30 minutes after the administration of Sodium Pertechnetate Tc 99m Injection.
The imaging of gastrointestinal bleeding is dependent on such factors as the region of imaging, rate and volume of the bleed, efficacy of the labeling of the red blood cells and timeliness of imaging. Due to these factors, images should be taken sequentially over a period of time until a positive image is obtained or clinical conditions warrant the discontinuance of the procedure. The period of time for collecting the images may range up to 36 hours.
Technetium Tc 99m Pyrophosphate Injection and the non-radioactive reconstituted Kit for the Preparation of Technetium Tc 99m Pyrophosphate Injection should be formulated within six (6) hours prior to clinical use.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term animal studies have been performed to evaluate carcinogenic potential or whether Technetium Tc 99m Pyrophosphate Injection affects fertility in males or females. Mutagenesis studies have not been conducted.
Pregnancy
Animal reproduction and teratogenicity studies have not been conducted with Technetium Tc 99m Pyrophosphate Injection. It is also not known whether Technetium Tc 99 Pyrophosphate Injection can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. Technetium Tc 99m Pyrophosphate Injection should be given to a pregnant woman only if clearly needed.
Ideally, examinations using radiopharmaceuticals, especially those elective in nature, to a woman of childbearing capability should be performed during the first few (approximately 10) days following the onset of menses.
Nursing mothers
Technetium Tc 99m Pyrophosphate Injection is excreted in human milk during lactation, therefore, formula feeding should be substituted for breast feeding.
Geriatric use
Clinical studies of the Kit for the Preparation of Technetium Tc 99m Pyrophosphate Injection did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
- ADVERSE REACTIONS
-
DOSAGE AND ADMINISTRATION
After preparation with oxidant-free Sodium Pertechnetate Tc 99m Injection, the suggested dose range of Technetium Tc 99m Pyrophosphate Injection in the average ADULT patient (70 kg) is:
Bone Imaging -185-555 megabecquerels (5-15 mCi)
Cardiac Imaging - 370-555 megabecquerels (10-15 mCi)
The suggested dose range of the non-radioactive reconstituted Kit for the Preparation of Technetium Tc 99m Pyrophosphate Injection in the average ADULT patient (70 kg) is:
Blood Imaging - Administer not less than one-third nor more than the total contents of one vial
[555-740 megabecquerels (15-20mCi) of Pertechnetate Tc 99m Injection].
Bone and Cardiac Imaging
Technetium Tc 99m Pyrophosphate Injection is injected intravenously over a 10 to 20 second period. For optimal results, bone imaging should be done 1 to 6 hours following administration. Cardiac imaging should be done 30 to 90 minutes following administration. The acute myocardial infarct can be visualized from 24 hours to 6 days following onset of symptoms, with maximum localization at 48 to 72 hours. Cardiac imaging should be done with a gamma scintillation camera. It is recommended that images be made of the anterior, left anterior oblique and left lateral projections.
Blood Pool Imaging
Kit for the Preparation of Technetium Tc 99m Pyrophosphate Injection may be reconstituted with sterile, non-pyrogenic isotonic saline containing no preservatives. Administer not less than one-third nor more than the total contents of one vial 30 minutes prior to the intravenous administration of 555 to 740 megabecquerels (15-20 mCi) Sodium Pertechnetate Tc 99m Injection. The non-radioactive reconstituted Kit for the Preparation of Technetium Tc 99m Pyrophosphate Injection should be injected by direct venipuncture. Heparinized catheter systems should be avoided. Cardiac imaging should be done 10 to 30 minutes following the administration of Sodium Pertechnetate Tc 99m Injection utilizing a scintillation camera interfaced to an electrocardiographic gating device.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
The patient dose should be measured by a suitable radioactivity calibration system immediately prior to administration.
Radiation Dosimetry
Bone and Cardiac Imaging
The effective half-life was assumed to be the physical half-life for all calculated values. The estimated radiation absorbed doses to an average ADULT patient (70 kg) from an intravenous injection of a maximum of 555 megabecquerels (15 mCi) of Technetium Tc 99m Pyrophosphate Injection are shown in Table 4.
TABLE 4: Estimated Absorbed Radiation Doses Bone and Cardiac Imaging* Technetium Tc 99m Pyrophosphate Injection
Target Organ
mGy/555 MBq
rads/15 mCi
Total Body
1.8
0.18
Kidneys
3.6
0.36
Red Marrow
3.5
0.35
Bone Surfaces
21.1
2.11
Bladder Wall
13.3
1.33
Testes
1.4
0.14
Ovaries
2.1
0.21
Effective Dose Equivalent
3.3 mSv
0.33 rem
*Based on the model in MIRD Dose Estimate Report No. 13 (J Nucl Med 30:1117-1122, 1989).
Estimate calculated using phantoms of Cristy & Eckerman (Report ORNL/TM-8381/V1 & V7). Bone and marrow model of Eckerman (Aspects of dosimetry of radionuclides within the skeleton with particular emphasis on the active marrow. In Fourth International Radiopharmaceutical Dosimetry Symposium; A.T. Schlafke-Stelson and E.E. Watson eds. CONF-851113, Oak Ridge Associated Universities, Oak Ridge, TN 37831, 1986. pp 514-534.) used.
The effective dose equivalent is a quantity which may be suitable for comparing risks of different procedures in nuclear medicine, radiology, and other applications involving ionizing radiation, but should not be construed to give information about risks to individual patients and should not be applied to situations involving radiation therapy.
Blood Pool Imaging
The estimated absorbed radiation doses to an average adult patient (70 kg) from an intravenous injection of 740 megabecquerels (20 mCi) of Sodium Pertechnetate Tc 99m Injection, 30 minutes after the intravenous administration of the non-radioactive reconstituted Kit for the Preparation of Technetium Tc 99m Pyrophosphate Injection are shown in Table 5.
TABLE 5: Estimated Absorbed Radiation Doses Blood Pool Imaginga Sodium Pertechnetate Tc 99m 30 min.
Post Injection with Pyrophosphate
Target Organ
mGy/740 MBq
rads/20 mCi
Total Body
3.2
0.32
Spleen
3.6
0.36
Bladder Wallb
24.0
2.40
Testes
2.4
0.24
Ovaries
4.6
0.46
Blood
10.4
1.04
Red Marrow
4.4
0.44
a Assume 75% of the Sodium Pertechnetate Tc 99m labels red blood cells and the other 25% remains as pertechnetate. Method of calculation: MIRD Dose Estimate Report No. 8, J Nucl Med. 17: 74-77, 1976.
b If 25% excreted with 1 hour Tb
-
HOW SUPPLIED
The Kit for the Preparation of Technetium Tc 99m Pyrophosphate Injection is supplied in packages of 5 or 30 sterile, non-pyrogenic, white capped 10mL vials.
Each multidose vial contains 12.0 mg sodium pyrophosphate, 2.8 mg minimum stannous tin as stannous chloride dihydrate and 4.9 mg maximum total tin as stannous chloride dihydrate; pH is adjusted with hydrochloric acid to 5.3-5.7 prior to lyophilization. No bacteriostatic preservative is present. Sealed under nitrogen.Included in each 5-vial package are one package insert and 10 radiation labels.
Included in each 30-vial package are one package insert and 60 radiation labels.
Store the kit as packaged at 20-25°C (68-77°F) [See USP]. Store the reconstituted vials at 20-25°C (68-77°) [See USP].
Bone and Cardiac Imaging
Technetium Tc 99m Pyrophosphate Injection is prepared from Kit for the Preparation of Technetium Tc 99m Pyrophosphate Injection by the following aseptic procedure:
- 1.
- Waterproof gloves should be worn during the preparation procedure. Remove the white flip-off cap from the vial and swab the top of the vial closure with alcohol to sterilize the surface.
- 2.
- Complete the radiation label and affix to the vial. Place the vial in an appropriate radiation shield suitably labeled and identified.
- 3.
- With a sterile shielded syringe, aseptically obtain 1-10 milliliters of a suitable, oxidant free, sterile and non-pyrogenic Sodium Pertechnetate Tc 99m Injection containing no more than 3.7 gigabecquerels (100 mCi). Aseptically add the Sodium Pertechnetate Tc 99m Injection to the vial.
- 4.
- Swirl the contents of the vial for one minute and let stand for at least 10 minutes.
- 5.
- Record date and time of preparation.
- 6.
- It is recommended that the radiochemical purity of the prepared radiopharmaceutical be checked prior to patient administration.
- 7.
- Examine vial contents for particulates and discoloration prior to injection.
- 8.
- Withdrawals for administration must be made aseptically using a sterile shielded syringe and needle. Since the vials contain nitrogen to prevent oxidation of the complex, the vials should not be vented. If repeated withdrawals are made from a vial, the replacement of contents with air should be minimized.
- 9.
- Aseptically withdraw material with a sterile lead shielded syringe for use within six (6) hours of preparation. For optimal results, this time should be minimized. The vial contains no bacteriostatic preservative. Store the reconstituted vial at 20-25°C (68-77°F) [See USP]. Discard the vial six (6) hours after reconstitution.
- 10.
- The patient dose should be measured by suitable radioactivity calibration system immediately prior to administration.
Blood Pool Imaging
The non-radioactive Kit for the Preparation of Technetium Tc 99m Pyrophosphate Injection is prepared by adhering to the following aseptic procedure:
- 1.
- Remove the white flip-off cap from the vial and swab the top of the vial closure with alcohol to sterilize the surface.
- 2.
- Reconstitute the reaction vial with 3 milliliters of sterile, non-pyrogenic, isotonic saline containing no preservatives.
- 3.
- Swirl the contents of the vial for one minute and let stand for at least 10 minutes.
- 4.
- Record date and time of preparation.
- 5.
- Examine vial contents for particulates and discoloration prior to injection.
- 6.
- Withdrawals for administration must be made aseptically using a sterile syringe and needle. Since the vials contain nitrogen to prevent oxidation of the complex, the vials should not be vented. If repeated withdrawals are made from a vial, the replacement of contents with air should be minimized.
- 7.
- Aseptically withdraw the reconstituted non-radioactive Kit for the Preparation of Technetium Tc 99m Pyrophosphate Injection with a sterile syringe for use within six (6) hours of preparation. For optimal results, this time should be minimized. The vial contains no bacteriostatic preservative. Store the reconstituted vial at 20-25°C (68-77°F) [See USP]. Discard the vial six (6) hours after reconstitution.
- 8.
- Between one-third and a total vial of stannous pyrophosphate may be administered by direct venipuncture 30 minutes prior to intravenous administration of 555 to 740 megabecquerels (15-20 mCi) of Sodium Pertechnetate Tc 99m Injection. Heparinized catheter systems should not be used.
- 9.
- The patient dose of Sodium Pertechnetate Tc 99m Injection should be measured by a suitable radioactivity calibration system immediately prior to administration.
NDC # 45567-0060-1 for 5 vial kits
NDC # 45567-0060-2 for 30 vial kits
This reagent kit for preparation of a radiopharmaceutical is approved for use by persons licensed pursuant to Section 120.547, Code of Massachusetts Regulation 105, or under equivalent license of the U.S. Nuclear Regulatory Commission or an Agreement State.
Manufactured By:
Sun Pharmaceutical Industries, Inc.
Billerica, MA 01821
PL-000017
Rev 1.2
Mar 2020
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL- VIAL CONTAINER
STERILE NON-PYROGENIC DIAGNOSTIC MULTIDOSE
KIT FOR THE PREPARTION OF TECHNETIUM Tc 99m PYROPHOSPHATE INJECTION
Each 10mL reaction vial contains in 12.0 mg sodium pyrophosphate, 2.8 mg minimum stannous tin as stannous chloride dihydrate and 4.9 mg maximum total tin as stannous chloride dehydrate; pH is adjusted to 5.3-5.7 with HCI prior to lyophilization. Sealed under nitrogen. Refer to Package Insert for directions for use and the recommended adult doses.
Manufactured by:
Sun Pharmaceutical Industries, Inc.
Billerica, MA 01821
PL-000018
Rev 0.2
Mar 2020
CONTAINS NO BACTERIOSTATIC PRESERVATIVE. FOR INTRAVENOUS
USE ONLY AFTER RECONSTITION WITH LABELING OXIDANT-FREE
TECHENTIUM Tc 99m or ISOTONIC SALINE. . Store at 20-25°C (68-77°F)[See USP] after reconstitution and use within 6 hours.
Rx Only
-
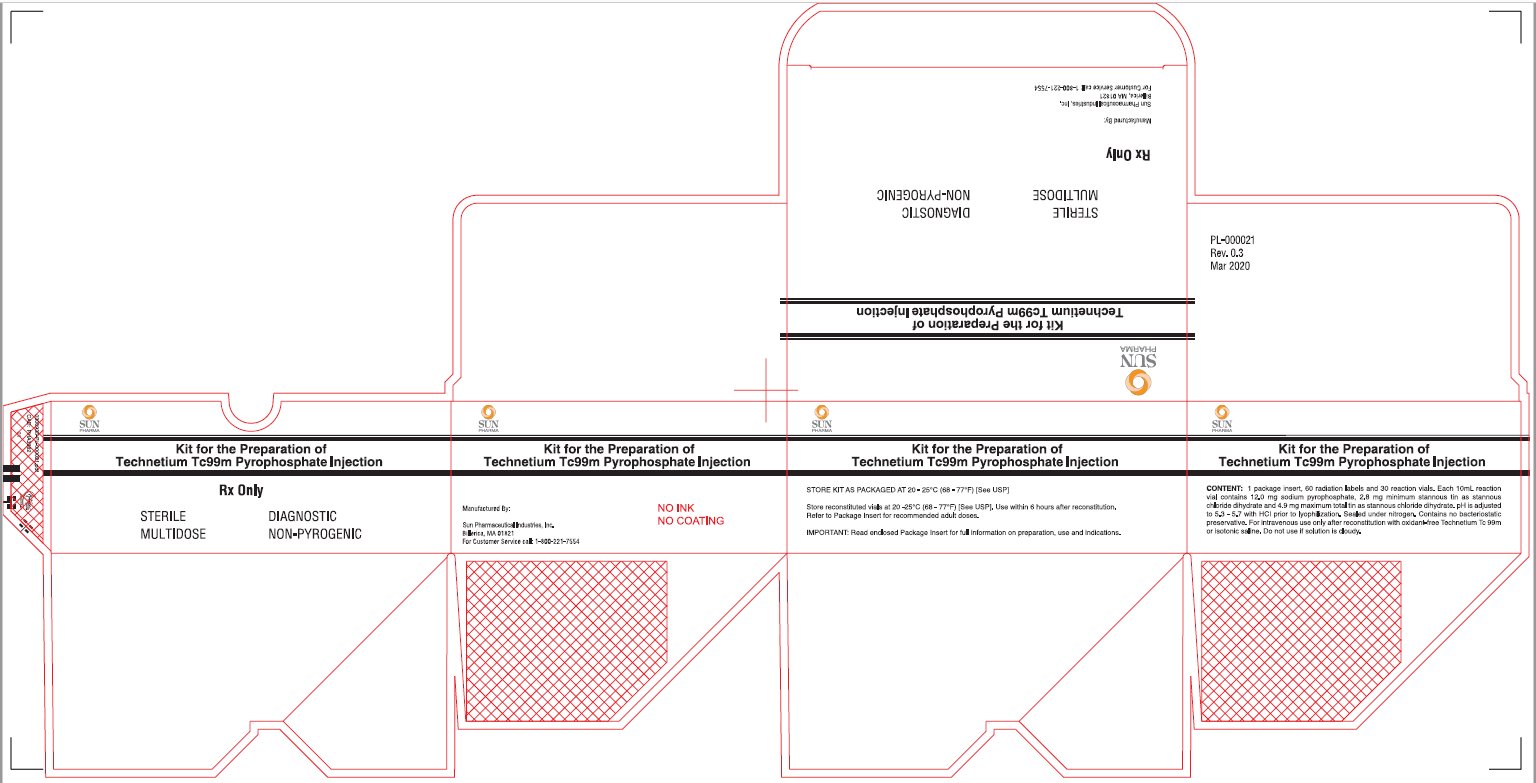
Package/Label Display Panel-5 VIAL BOX
Kit for the Preparation of Technetium Tc99m Pyrophosphate Injection
Manufactured By:
Sun Pharmaceutical Industries, Inc.
Billerica, MA 01821
For Customer Service call: 1-800-221-7554
CONTENTS:
1 package insert, 10 radiation labels and 5 reaction vials. Each 10 mL reaction vial contains 12.0 mg sodium pyrophosphate, 2.8 mg minimum stannous tin as stannous chloride dehydrate and 4.9 mg maximum total tin as stannous chloride deihydrate. pH is adjusted to 5.3 – 5.7 with HCI prior to lyophilization. Sealed under nitrogen. Contains no bacteriostatic preservative. For intravenous use only after reconstitution with oxidant-free Technetium Tc 99m or isotonic saline. Do not use if solution is cloudy.
STORE KIT AS PACKAGED AT 20-25°C (68-77°F) [SEE USP]
Store reconstituted vials at 20-25°C (68-77°F) [See USP]. Use within 6 hours after reconstitution. Refer to Package Insert for recommended adult doses.
IMPORTANT: Read enclosed Package Insert for full information on preparation, use and indications.
Manufactured By:
Sun Pharmaceutical Industries, Inc.
Billerica, MA 01821
For Customer Service call: 1-800-221-7554
PL-000020
Rev 0.3
Mar 2020
-
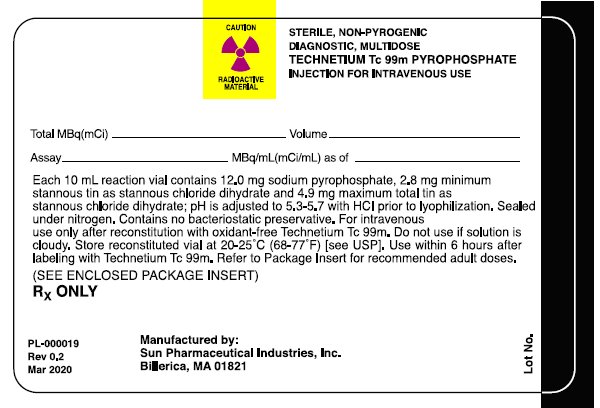
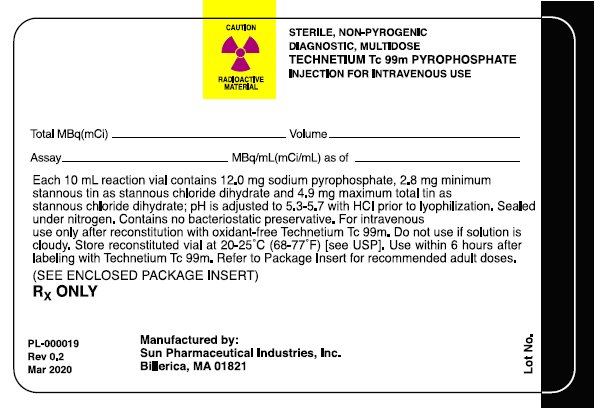
Package/Label Display Panel- Radiation Label
CAUTION RADIOACTIVE MATERIAL
STERILE, NON-PYROGENIC, DIAGNOSTIC, MULTIDOSE TECHNETIUM Tc 99m PYROPHOSPHATE INJECTION FOR INTRAVENOUS USE
Total MBq (mCi)_____Volume_____
Assay_____MBq/mL(mCi/mL) as of _____
Each 10mL reaction vial contains 12.0 mg sodium pyrophosphate, 2.8 mg minimum stannous tin as stannous chloride dihydrate and 4.9 mg maximum total tin as stannous chloride dihydrate; pH is adjusted to 5.3-5.7 with HCI prior to lyophilization. Sealed under nitrogen. Contains no bacteriostatic preservative. For intravenous use only after reconstitution with oxidant-free Technetium Tc 99m. Do not use if solution is cloudy. Store reconstituted vial at 20-25°C (68-77°F) [see USP]. Use within 6 hours after labeling with Technetium Tc 99m. Refer to Package Insert for recommended adult doses.
(SEE ENCLOSED PACKAGE INSERT)
RX ONLY
Manufactured By:
Sun Pharmaceutical Industries, Inc.
Billerica, MA 01821
PL-000019
Rev 0.2
Mar 2020
-
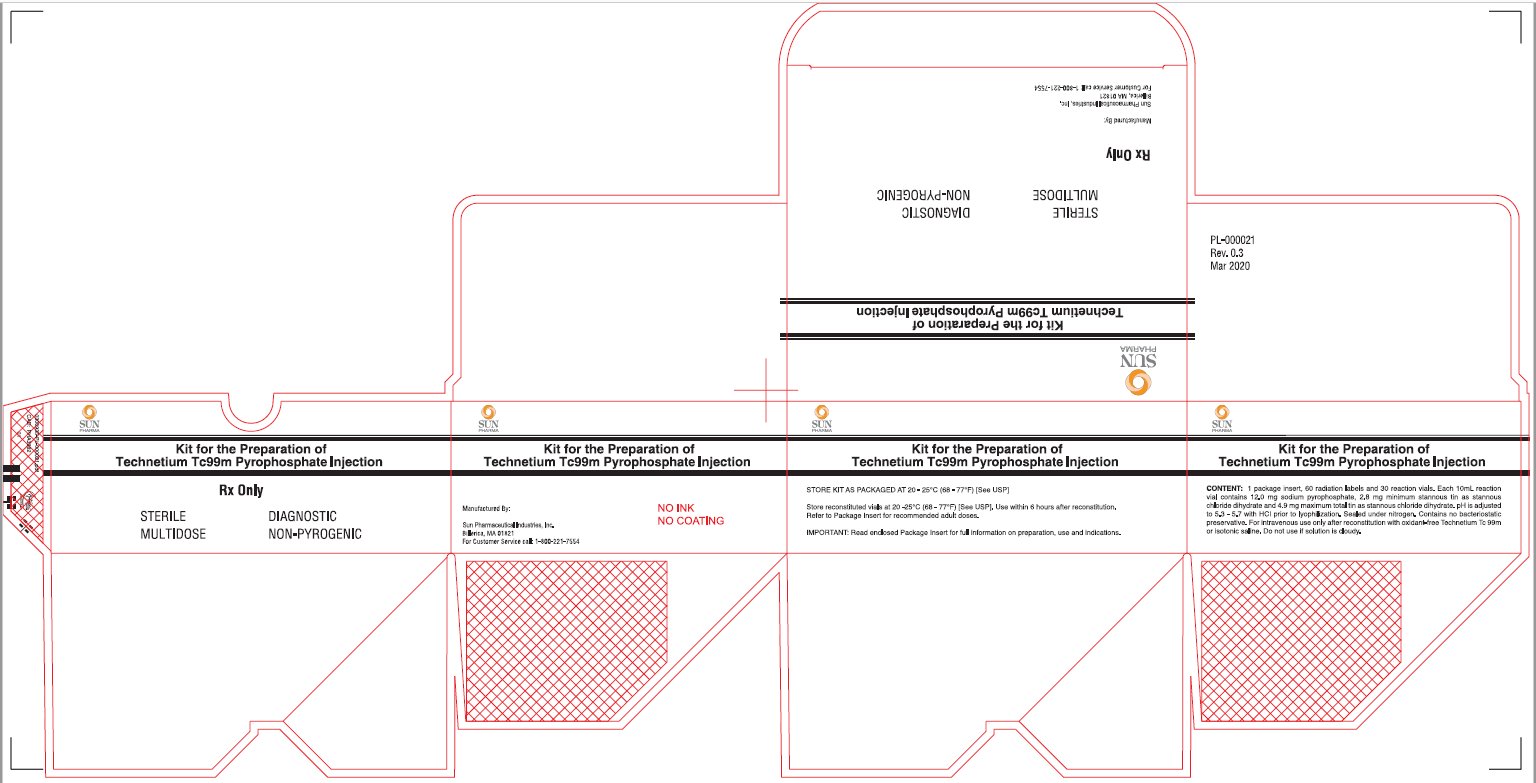
Package/Label Display Panel-30 Vial Box
Kit for the preparation of Technetium Tc99m Pyrophosphate Injection
STERILE, NON-PYROGENIC, DIAGNOSTIC, MULTIDOSE
Rx only.
Manufactured By:
Sun Pharmaceutical Industries, Inc.
Billerica, MA 01821
For Customer Service call: 1-800-221-7554
Sterile Diagnostic Multidose Non-Pyrogenic
CONTENTS: 1 Package insert, 60 radiation labels and 30 reaction vials. Each 10 mL vial contains 12.0 mg sodium pyrophosphate, 2.8 mg minimum stannous tin as stannous chloride dihydrate and 4.9 mg maximum total tin as stannous chloride dihydrate. pH is adjusted to 5.3 – 5.7 with HCI prior to lyophilization. Sealed under nitrogen. Contains no bacteriostatic preservative. For intravenous use only after reconstitution with oxidant-free Technetium Tc 99m or isotonic saline. Do not use if solution is cloudy.
Store the kit as packaged AT 20-25°C (68-77°F) [See USP]
Store reconstituted vials at 20-25°C (68-77°F) [See USP]. Use within 6 hours after reconstitution.
Refer to Package Insert for recommended adult doses.
IMPORTANT: Read enclosed Package Insert for full information on preparation, use and indications.
Manufactured By:
Sun Pharmaceutical Industries, Inc.
Billerica, MA 01821
PL-000021
Rev 0.3
Mar 2020
-
INGREDIENTS AND APPEARANCE
KIT FOR THE PREPARATION OF TECHNETIUM TC99M PYROPHOSPHATE
technetium tc99m pyrophosphate injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:45567-0060 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TECHNETIUM TC-99M PYROPHOSPHATE (UNII: 5L76I61H2B) (TECHNETIUM TC-99M PYROPHOSPHATE - UNII:5L76I61H2B) SODIUM PYROPHOSPHATE 12 mg in 10 mL Inactive Ingredients Ingredient Name Strength STANNOUS CHLORIDE (UNII: 1BQV3749L5) 2.8 mg in 10 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:45567-0060-1 5 in 1 KIT 06/30/1987 1 10 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product 2 NDC:45567-0060-2 30 in 1 KIT 06/30/1987 2 10 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019039 06/30/1987 Labeler - Sun Pharmaceutical Industries, Inc. (139261648) Registrant - Sun Pharmaceutical Industries, Inc. (139261648) Establishment Name Address ID/FEI Business Operations Sun Pharmaceutical Industries, Inc. 139261648 ANALYSIS(45567-0060) , MANUFACTURE(45567-0060) , PACK(45567-0060)