NYSTATIN- nystatin suspension
Medley Pharmaceuticals Limited
----------
Nystatin Oral Suspension, USP (100,000 units per mL)
1 INDICATIONS & USAGE
Nystatin oral suspension is indicated for the treatment of infections of the oral cavity caused by Candida albicans.
2 DOSAGE & ADMINISTRATION
Infants: 2 mL (200,000 units) four times daily (1 mL in each side of mouth).
Pediatric patients and adults: 4 to 6 mL (400,000 to 600,000 units) four times daily (one-half of dose in each side of mouth).
NOTE: Limited clinical studies in neonates, including premature and low-birth weight neonates, indicate that 1 mL (100,000 units) four times daily is effective.
Local treatment should be continued at least 48 hours after perioral symptoms have disappeared and/or cultures returned to normal. It is recommended that the drug be retained in the mouth as long as possible before swallowing.
CAUTION
The Packaging of This Product Contains Natural Rubber Latex Which May Cause Allergic Reactions
4 CONTRAINDICATIONS
Nystatin is contraindicated in patients with a history of hypersensitivity to nystatin or any of the suspension components
5 PRECAUTIONS
General
Discontinue treatment with nystatin if sensitization or irritation is reported during use.
Nystatin is not effective in the treatment of systemic mycoses since it is not significantly absorbed from the gastrointestinal tract.
Information for the Patient
Patient should be advised to retain nystatin in the mouth as long as possible and to continue its use for at least 2 days after symptoms have subsided.
There should be no interruption or discontinuation of the medication until the prescribed course of treatment is completed, even though symptomatic relief may occur within a few days.
If symptoms of local irritation develop, the physician should be notified immediately.
Laboratory Tests
If there is a lack of therapeutic response, appropriate microbiological studies (e .g., KOH smears and/or cultures) should be repeated to confirm the diagnosis of candidiasis and rule out other pathogens before instituting another course of therapy.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term animal studies have been performed to evaluate the carcinogenic potential of nystatin. In mice exposed to nystatin 50 mg/kg by injection, an increased incidence of chromosomal aberrations, consisting primarily of chromatid breaks, was observed in bone marrow cells. However, there have been no studies to determine the mutagenicity of orally-administered nystatin or its effects on fertility in males or females.
Pregnancy :
Teratogenic Effects
Teratogenicity studies have not been conducted with nystatin. It is also not known whether nystatin can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Nystatin should be given to a pregnant woman only if clearly needed.
Nonteratogenic Effects
In one rat reproductive study, nystatin was administered orally to pregnant rats in single doses of 100,500, or 3000 mg/kg on the ninth day of gestation, or as multiple doses of 500 mg/kg/day on gestation days 1-20, 1-4, 7-10, 11-14, or 15-18. It was found that nystatin had a slight abortive effect when used during the whole period of pregnancy. No abnormalities were seen in surviving fetuses. Although no adverse effects or complications have been attributed to the use of intra-vaginal nystatin in neonates born to women treated during pregnancy, no similar studies evaluating complications of oral nystatin have been conducted.
Nursing Mothers
It is not known whether nystatin is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when nystatin is administered to a nursing woman.
Pediatric Use
See
DOSAGE AND ADMINISTRATION section for pediatric dosing recommendations.
6 ADVERSE REACTIONS
To report SUSPECTED ADVERSE REACTIONS, contact FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
Gastrointestinal symptoms including diarrhea, gastrointestinal distress, nausea, vomiting and burning of the mouth have been reported. Hypersensitivity reactions including rash, pruritus, and anaphylactoid reaction have also been reported.
10 OVERDOSAGE
Oral doses of nystatin in excess of five million units daily have caused nausea and gastrointestinal upset.
11 DESCRIPTION
Nystatin is obtained from Streptomyces noursei. It is known to be a mixture, but the composition has not been completely elucidated. Nystatin A is closely related to amphotericin B. Each is a macro-cyclic lactone containing a ketal ring, an all-trans polyene system, and a mycosamine (3-amino-3-deoxyrhamose) moiety.
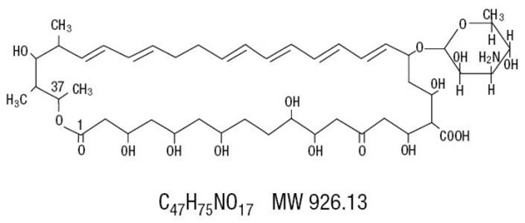
Nystatin Oral Suspension, for oral administration, contains 100,000 USP Nystatin Units per mL. Inactive ingredients: alcohol (≤ 1% v/v), sucrose 50% w/v, peppermint oil, NF, cinnamaldehyde, disodium hydrogen phosphate,USP, carboxymethylcellulose sodium, USP, glycerin, USP, saccharin sodium, USP, cherry flavor, methylparaben, NF,propylparaben, NF and purified water,USP. May also contain sodium hydroxide, NF and/ or hydrochloric acid, NF for pH adjustment.
12 CLINICAL PHARMACOLOGY
Nystatin acts by binding to sterols in the cell membrane of the fungus with a resultant change in membrane permeability allowing leakage of intracellular components. Nystatin is absorbed very sparingly following oral administration, with no detectable blood levels when given in the recommended doses.
16 HOW SUPPLIED/STORAGE AND HANDLING
Nystatin Oral Suspension, USP, 100,000 USP Nystatin Units per mL, is available as a cherry-mint flavored, light creamy yellow, ready-to-use suspension, in the following sizes:
60 mL bottles with a
child-resistant cap and calibrated dropper.
1 Pint (473 mL) bottles with a child-resistant cap
Storage
This package is child-resistant. Keep out of reach of children
. Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Avoid freezing
NDC for 60 ml: 42934-400-51
NDC for 473 ml : 42934-400-52
Rx Only
Manufactured For:
Medley Pharmaceuticals Ltd.
Medley House, D2, M.I.D.C Area,
16th Road, Andheri (East), Mumbai-400093, INDIA.
Manufactured By:
Medley Pharmaceuticals Ltd.
Plot No. 18 and 19, Survey No. 378 / 7 & 8, 379 / 2 & 3,
Zari Causeway Road, Kachigam, Daman - 396210, INDIA.
REV. 10-21
| NYSTATIN
nystatin suspension |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - Medley Pharmaceuticals Limited (677602480) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Medley Pharmaceuticals Limited | 677602480 | manufacture(42934-400) | |


