ARISULFUR ACNE TREATMENT- sulfur soap
A+Plus Medical Center Limited
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
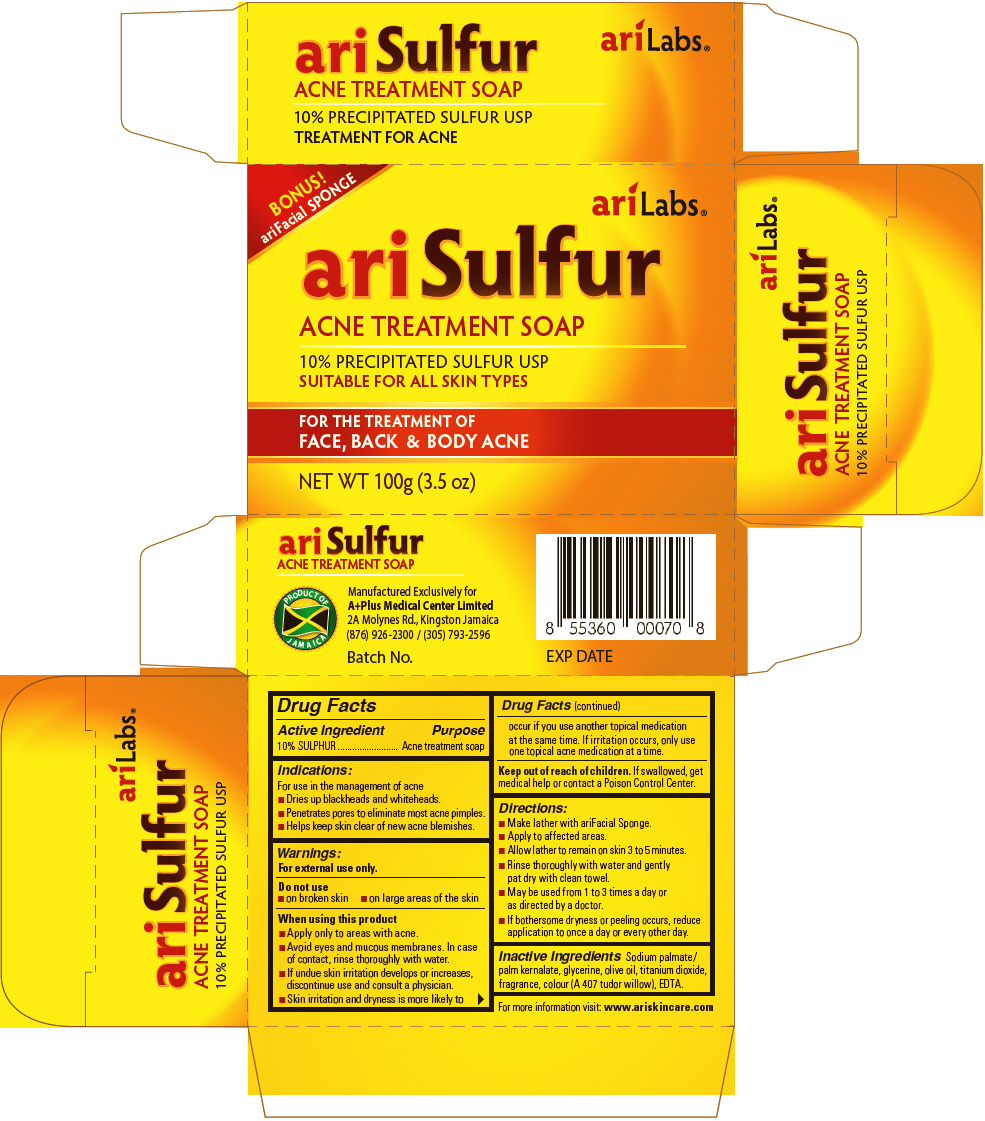
ariSulfur
Acne Treatment Soap
Indications
For use in the management of acne
- Dries up blackheads and whiteheads.
- Penetrates pores to eliminate most acne pimples.
- Helps keep skin clear of new acne blemishes.
Warnings
For external use only.
When using this product
- Apply only to areas with acne.
- Avoid eyes and mucous membranes. In case of contact, rinse thoroughly with water.
- If undue skin irritation develops or increases, discontinue use and consult a physician.
- Skin irritation and dryness is more likely to occur if you use another topical medication at the same time. If irritation occurs, only use one topical acne medication at a time.
Directions
- Make lather with ariFacial Sponge.
- Apply to affected areas.
- Allow lather to remain on skin 3 to 5 minutes.
- Rinse thoroughly with water and gently pat dry with clean towel.
- May be used from 1 to 3 times a day or as directed by a doctor.
- If bothersome dryness or peeling occurs, reduce application to once a day or every other day.
| ARISULFUR
ACNE TREATMENT
sulfur soap |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - A+Plus Medical Center Limited (873273726) |
Revised: 12/2018
Document Id: 22974693-5b61-4b9f-864f-73fd598d1050
Set id: 1c72e7f6-fc28-4841-a366-f303f405a940
Version: 2
Effective Time: 20181219
A+Plus Medical Center Limited