GENVOYA- elvitegravir, cobicistat, emtricitabine, and tenofovir alafenamide tablet
A-S Medication Solutions
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use GENVOYA safely and effectively. See full prescribing information for GENVOYA.
GENVOYA® (elvitegravir, cobicistat, emtricitabine, and tenofovir alafenamide) tablets, for oral use Initial U.S. Approval: 2015 WARNING: POST TREATMENT ACUTE EXACERBATION OF HEPATITIS BSee full prescribing information for complete boxed warning.Severe acute exacerbations of hepatitis B have been reported in patients who are coinfected with HIV-1 and HBV and have discontinued products containing emtricitabine and/or tenofovir disoproxil fumarate (TDF), and may occur with discontinuation of GENVOYA. Hepatic function should be monitored closely in these patients. If appropriate, anti-hepatitis B therapy may be warranted. (5.1) RECENT MAJOR CHANGES
INDICATIONS AND USAGEGENVOYA is a four-drug combination of elvitegravir, an HIV-1 integrase strand transfer inhibitor (INSTI), cobicistat, a CYP3A inhibitor, and emtricitabine and tenofovir alafenamide (TAF), both HIV-1 nucleoside analog reverse transcriptase inhibitors (NRTIs), and is indicated as a complete regimen for the treatment of HIV-1 infection in adults and pediatric patients weighing at least 25 kg who have no antiretroviral treatment history or to replace the current antiretroviral regimen in those who are virologically-suppressed (HIV-1 RNA less than 50 copies per mL) on a stable antiretroviral regimen for at least 6 months with no history of treatment failure and no known substitutions associated with resistance to the individual components of GENVOYA. (1) DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHSTablets: 150 mg of elvitegravir, 150 mg of cobicistat, 200 mg of emtricitabine, and 10 mg of tenofovir alafenamide. (3) CONTRAINDICATIONSCoadministration of GENVOYA is contraindicated with drugs that: WARNINGS AND PRECAUTIONS
ADVERSE REACTIONSMost common adverse reaction (incidence greater than or equal to 10%, all grades) is nausea. (6.1) To report SUSPECTED ADVERSE REACTIONS, contact Gilead Sciences, Inc. at 1-800-GILEAD-5 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling. Revised: 1/2020 |
FULL PRESCRIBING INFORMATION
WARNING: POST TREATMENT ACUTE EXACERBATION OF HEPATITIS B
Severe acute exacerbations of hepatitis B have been reported in patients who are coinfected with HIV-1 and HBV and have discontinued products containing emtricitabine and/or tenofovir disoproxil fumarate (TDF), and may occur with discontinuation of GENVOYA. Hepatic function should be monitored closely with both clinical and laboratory follow-up for at least several months in patients who are coinfected with HIV-1 and HBV and discontinue GENVOYA. If appropriate, anti-hepatitis B therapy may be warranted [see Warnings and Precautions (5.1)].
1 INDICATIONS AND USAGE
GENVOYA is indicated as a complete regimen for the treatment of HIV-1 infection in adults and pediatric patients weighing at least 25 kg who have no antiretroviral treatment history or to replace the current antiretroviral regimen in those who are virologically-suppressed (HIV-1 RNA less than 50 copies per mL) on a stable antiretroviral regimen for at least 6 months with no history of treatment failure and no known substitutions associated with resistance to the individual components of GENVOYA [see Clinical Studies (14)].
2 DOSAGE AND ADMINISTRATION
2.1 Testing When Initiating and During Treatment with GENVOYA
Prior to or when initiating GENVOYA, test patients for hepatitis B virus infection [see Warnings and Precautions (5.1)].
Prior to or when initiating GENVOYA, and during treatment with GENVOYA on a clinically appropriate schedule, assess serum creatinine, estimated creatinine clearance, urine glucose and urine protein in all patients. In patients with chronic kidney disease, also assess serum phosphorus [see Warnings and Precautions (5.4)].
2.2 Recommended Dosage
GENVOYA is a four-drug fixed dose combination product containing 150 mg of elvitegravir, 150 mg of cobicistat, 200 mg of emtricitabine, and 10 mg of tenofovir alafenamide (TAF). The recommended dosage of GENVOYA is one tablet taken orally once daily with food in:
- adults and pediatric patients with body weight at least 25 kg and creatinine clearance greater than or equal to 30 mL per minute; or
- adults with creatinine clearance below 15 mL per minute who are receiving chronic hemodialysis. On days of hemodialysis, administer GENVOYA after completion of hemodialysis treatment [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)].
2.3 Not Recommended in Patients with Severe Renal Impairment
GENVOYA is not recommended in patients with:
- severe renal impairment (estimated creatinine clearance of 15 to below 30 mL per minute); or
- end stage renal disease (ESRD; estimated creatinine clearance below 15 mL per minute) who are not receiving chronic hemodialysis [see Dosage and Administration (2.2) and Use in Specific Populations (8.6)].
2.4 Not Recommended in Patients with Severe Hepatic Impairment
GENVOYA is not recommended in patients with severe hepatic impairment (Child-Pugh Class C) [see Use in Specific Populations (8.7) and Clinical Pharmacology (12.3)].
2.5 Not Recommended During Pregnancy
GENVOYA is not recommended for use during pregnancy because of substantially lower exposures of cobicistat and elvitegravir during the second and third trimesters [see Use in Specific Populations (8.1)].
GENVOYA should not be initiated in pregnant individuals. An alternative regimen is recommended for individuals who become pregnant during therapy with GENVOYA [see Use in Specific Populations (8.1)].
3 DOSAGE FORMS AND STRENGTHS
Each GENVOYA tablet contains 150 mg of elvitegravir, 150 mg of cobicistat, 200 mg of emtricitabine, and 10 mg of tenofovir alafenamide (TAF) (equivalent to 11.2 mg of tenofovir alafenamide fumarate).
The tablets are green, capsule-shaped, film-coated tablets, debossed with "GSI" on one side of the tablet and the number "510" on the other side of the tablet.
4 CONTRAINDICATIONS
Coadministration of GENVOYA is contraindicated with drugs that are highly dependent on CYP3A for clearance and for which elevated plasma concentrations are associated with serious and/or life-threatening events. These drugs and other contraindicated drugs (which may lead to reduced efficacy of GENVOYA and possible resistance) are listed below [see Drug Interactions (7.5) and Clinical Pharmacology (12.3)].
- Alpha 1-adrenoreceptor antagonist: alfuzosin
- Anticonvulsants: carbamazepine, phenobarbital, phenytoin
- Antimycobacterial: rifampin
- Antipsychotics: lurasidone, pimozide
- Ergot Derivatives: dihydroergotamine, ergotamine, methylergonovine
- GI Motility Agent: cisapride
- Herbal Products: St. John's wort (Hypericum perforatum)
- Lipid-modifying Agents: lomitapide, lovastatin, simvastatin
- Phosphodiesterase-5 (PDE-5) Inhibitor: sildenafil when administered as REVATIO® for the treatment of pulmonary arterial hypertension
- Sedative/hypnotics: triazolam, orally administered midazolam
5 WARNINGS AND PRECAUTIONS
5.1 Severe Acute Exacerbation of Hepatitis B in Patients Coinfected with HIV-1 and HBV
Patients with HIV-1 should be tested for the presence of hepatitis B virus (HBV) before or when initiating antiretroviral therapy [see Dosage and Administration (2.1)].
Severe acute exacerbations of hepatitis B (e.g., liver decompensation and liver failure) have been reported in patients who are coinfected with HIV-1 and HBV and have discontinued products containing emtricitabine and/or tenofovir disoproxil fumarate (TDF), and may occur with discontinuation of GENVOYA. Patients coinfected with HIV-1 and HBV who discontinue GENVOYA should be closely monitored with both clinical and laboratory follow-up for at least several months after stopping treatment. If appropriate, anti-hepatitis B therapy may be warranted, especially in patients with advanced liver disease or cirrhosis, since post-treatment exacerbation of hepatitis may lead to hepatic decompensation and liver failure.
5.2 Risk of Adverse Reactions or Loss of Virologic Response Due to Drug Interactions
The concomitant use of GENVOYA and other drugs may result in known or potentially significant drug interactions, some of which may lead to [see Contraindications (4) and Drug Interactions (7.5)]:
- Loss of therapeutic effect of GENVOYA and possible development of resistance.
- Possible clinically significant adverse reactions from greater exposures of concomitant drugs.
See Table 5 for steps to prevent or manage these possible and known significant drug interactions, including dosing recommendations. Consider the potential for drug interactions prior to and during GENVOYA therapy; review concomitant medications during GENVOYA therapy; and monitor for the adverse reactions associated with the concomitant drugs.
5.3 Immune Reconstitution Syndrome
Immune reconstitution syndrome has been reported in patients treated with combination antiretroviral therapy, including emtricitabine, a component of GENVOYA. During the initial phase of combination antiretroviral treatment, patients whose immune system responds may develop an inflammatory response to indolent or residual opportunistic infections [such as Mycobacterium avium infection, cytomegalovirus, Pneumocystis jirovecii pneumonia (PCP), or tuberculosis], which may necessitate further evaluation and treatment.
Autoimmune disorders (such as Graves' disease, polymyositis, and Guillain-Barré syndrome) have also been reported to occur in the setting of immune reconstitution, however, the time to onset is more variable, and can occur many months after initiation of treatment.
5.4 New Onset or Worsening Renal Impairment
Renal impairment, including cases of acute renal failure and Fanconi syndrome (renal tubular injury with severe hypophosphatemia), has been reported with the use of tenofovir prodrugs in both animal toxicology studies and human trials. In clinical trials of GENVOYA, there have been no cases of Fanconi syndrome or Proximal Renal Tubulopathy (PRT). In clinical trials of GENVOYA in treatment-naïve subjects and in virologically suppressed subjects switched to GENVOYA with estimated creatinine clearance greater than 50 mL per minute, renal serious adverse events or discontinuations due to renal adverse reactions were encountered in less than 1% of participants treated with GENVOYA. In a study of virologically suppressed subjects with baseline estimated creatinine clearance between 30 and 69 mL per minute treated with GENVOYA for a median duration of 144 weeks, GENVOYA was permanently discontinued due to worsening renal function in three of 80 (4%) subjects with a baseline estimated creatinine clearance between 30 and 50 mL per minute and two of 162 (1%) with a baseline estimated creatinine clearance greater than or equal to 50 mL per minute [see Adverse Reactions (6.1)]. GENVOYA is not recommended in patients with estimated creatinine clearance of 15 to below 30 mL per minute, or in patients with estimated creatinine clearance below 15 mL per minute who are not receiving chronic hemodialysis.
Patients taking tenofovir prodrugs who have impaired renal function and those taking nephrotoxic agents including non-steroidal anti-inflammatory drugs are at increased risk of developing renal-related adverse reactions.
Prior to or when initiating GENVOYA, and during treatment with GENVOYA on a clinically appropriate schedule, assess serum creatinine, estimated creatinine clearance, urine glucose and urine protein in all patients. In patients with chronic kidney disease, also assess serum phosphorus. Discontinue GENVOYA in patients who develop clinically significant decreases in renal function or evidence of Fanconi syndrome.
Cobicistat, a component of GENVOYA, produces elevations of serum creatinine due to inhibition of tubular secretion of creatinine without affecting glomerular filtration [see Adverse Reactions (6.1)]. The elevation is typically seen within 2 weeks of starting therapy and is reversible after discontinuation. Patients who experience a confirmed increase in serum creatinine of greater than 0.4 mg per dL from baseline should be closely monitored for renal safety.
5.5 Lactic Acidosis/Severe Hepatomegaly with Steatosis
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogs, including emtricitabine, a component of GENVOYA, and tenofovir DF, another prodrug of tenofovir, alone or in combination with other antiretrovirals. Treatment with GENVOYA should be suspended in any patient who develops clinical or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity (which may include hepatomegaly and steatosis even in the absence of marked transaminase elevations).
6 ADVERSE REACTIONS
The following adverse drug reactions are discussed in other sections of the labeling:
- Severe Acute Exacerbations of Hepatitis B [see Boxed Warning and Warnings and Precautions (5.1)]
- Immune Reconstitution Syndrome [see Warnings and Precautions (5.3)]
- New Onset or Worsening Renal Impairment [see Warnings and Precautions (5.4)]
- Lactic Acidosis/Severe Hepatomegaly with Steatosis [see Warnings and Precautions (5.5)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Clinical Trials in Treatment-Naïve Adults
The primary safety assessment of GENVOYA was based on Week 144 pooled data from 1,733 subjects in two randomized, double-blind, active-controlled trials, Study 104 and Study 111, in antiretroviral treatment-naïve HIV-1 infected adult subjects. A total of 866 subjects received one tablet of GENVOYA once daily [see Clinical Studies (14.2)].
The most common adverse reaction (all Grades) reported in at least 10% of subjects in the GENVOYA group was nausea. The proportion of subjects who discontinued treatment with GENVOYA or STRIBILD® due to adverse events, regardless of severity, was 1% and 2%, respectively. Table 1 displays the frequency of adverse reactions (all Grades) greater than or equal to 5% in the GENVOYA group.
| GENVOYA N=866 | STRIBILD N=867 |
|
|---|---|---|
|
||
| Nausea | 11% | 13% |
| Diarrhea | 7% | 9% |
| Headache | 6% | 5% |
| Fatigue | 5% | 4% |
The majority of events presented in Table 1 occurred at severity Grade 1.
Clinical Trials in Virologically Suppressed Adults
The safety of GENVOYA in virologically-suppressed adults was based on Week 96 data from 959 subjects in a randomized, open-label, active-controlled trial (Study 109) in which virologically-suppressed subjects were switched from a TDF-containing combination regimen to GENVOYA. Overall, the safety profile of GENVOYA in subjects in this study was similar to that of treatment-naïve subjects [see Clinical Studies (14.3)]. Additional adverse reactions observed with GENVOYA in Study 109 included suicidal ideation, suicidal behavior, and suicide attempt (<1% combined); all of these events were serious and all occurred in subjects with a preexisting history of depression or psychiatric illness.
Clinical Trials in Adult Subjects with Renal Impairment
In an open-label trial (Study 112), 248 HIV-1 infected subjects with estimated creatinine clearance between 30 and 69 mL per minute (by Cockcroft-Gault method) were treated with GENVOYA for a median duration of 144 weeks. Of these subjects, 65% had previously been on a stable TDF-containing regimen. A total of 5 subjects permanently discontinued GENVOYA due to the development of renal adverse events through Week 96. Three of these five were among the 80 subjects with baseline estimated creatinine clearance of less than 50 mL/min and two subjects were among the 162 subjects with baseline estimated creatinine clearance of greater than or equal to 50 mL/min. There were no further renal discontinuations between Weeks 96 and 144. Overall, renally impaired subjects receiving GENVOYA in this study had a mean serum creatinine of 1.5 mg/dL at baseline and 1.4 mg/dL at Week 144. Otherwise, the safety profile of GENVOYA in subjects in this study was similar to that of subjects with normal renal function.
Virologically-Suppressed Adults with End Stage Renal Disease (ESRD) Receiving Chronic Hemodialysis
The safety of GENVOYA in subjects with end stage renal disease (ESRD) (estimated creatinine clearance of less than 15 mL/min) on chronic hemodialysis was assessed in 55 subjects (Study 1825) [see Clinical Studies (14.4)]. The most commonly reported adverse reaction (adverse event assessed as causally related by investigator and all grades) was nausea (7%). Serious adverse events were reported in 53% of subjects and the most common serious adverse events were pneumonia (13%), fluid overload (7%), hyperkalemia (7%) and osteomyelitis (7%). Overall 5% of subjects permanently discontinued treatment due to an adverse event.
Renal Laboratory Tests and Renal Safety
Treatment-Naïve Adults:
Cobicistat (a component of GENVOYA) has been shown to increase serum creatinine due to inhibition of tubular secretion of creatinine without affecting glomerular filtration [see Clinical Pharmacology (12.2)]. Increases in serum creatinine occurred by Week 2 of treatment and remained stable through 144 weeks.
In two 144-week randomized, controlled trials in a total of 1,733 treatment-naïve adults with a median baseline estimated creatinine clearance of 115 mL per minute, mean serum creatinine increased by less than 0.1 mg per dL in the GENVOYA group and by 0.1 mg per dL in the STRIBILD group from baseline to Week 144.
Virologically Suppressed Adults:
In a study of 1,436 virologically-suppressed TDF-treated adults with a mean baseline estimated creatinine clearance of 112 mL per minute who were randomized to continue their treatment regimen or switch to GENVOYA, at Week 96 mean serum creatinine was similar to baseline for both those continuing baseline treatment and those switching to GENVOYA.
Bone Mineral Density Effects
Treatment-Naïve Adults:
In a pooled analysis of Studies 104 and 111, the effects of GENVOYA compared to STRIBILD on bone mineral density (BMD) change from baseline to Week 144 were assessed by dual-energy X-ray absorptiometry (DXA). The mean percentage change in BMD from baseline to Week 144 was −0.92% with GENVOYA compared to −2.95% with STRIBILD at the lumbar spine and −0.75% compared to −3.36% at the total hip. BMD declines of 5% or greater at the lumbar spine were experienced by 15% of GENVOYA subjects and 29% of STRIBILD subjects. BMD declines of 7% or greater at the femoral neck were experienced by 15% of GENVOYA subjects and 29% of STRIBILD subjects. The long-term clinical significance of these BMD changes is not known.
Virologically Suppressed Adults:
In Study 109, TDF-treated subjects were randomized to continue their TDF-based regimen or switch to GENVOYA; changes in BMD from baseline to Week 96 were assessed by DXA. Mean BMD increased in subjects who switched to GENVOYA (2.12% lumbar spine, 2.44% total hip) and decreased slightly in subjects who continued their baseline regimen (−0.09% lumbar spine, −0.46% total hip). BMD declines of 5% or greater at the lumbar spine were experienced by 2% of GENVOYA subjects and 6% of subjects who continued their TDF-based regimen. BMD declines of 7% or greater at the femoral neck were experienced by 2% of GENVOYA subjects and 7% of subjects who continued their TDF-based regimen. The long-term clinical significance of these BMD changes is not known.
Laboratory Abnormalities:
The frequency of laboratory abnormalities (Grades 3–4) occurring in at least 2% of subjects receiving GENVOYA in Studies 104 and 111 are presented in Table 2.
| Laboratory Parameter Abnormality* | GENVOYA N=866 | STRIBILD N=867 |
|---|---|---|
|
||
| Creatine Kinase (≥10.0 × ULN) | 11% | 10% |
| LDL-cholesterol (fasted) (>190 mg/dL) | 11% | 5% |
| Total cholesterol (fasted) (>300mg/dL) | 4% | 3% |
| Amylase | 3% | 5% |
| ALT | 3% | 3% |
| AST | 3% | 4% |
| Urine RBC (Hematuria) (>75 RBC/HPF) | 3% | 3% |
Serum Lipids:
Subjects receiving GENVOYA experienced greater increases in serum lipids compared to those receiving STRIBILD.
Changes from baseline in total cholesterol, HDL-cholesterol, LDL-cholesterol, triglycerides and total cholesterol to HDL ratio are presented in Table 3.
| GENVOYA N=866 | STRIBILD N=867 |
|||
|---|---|---|---|---|
| Baseline | Week 144 | Baseline | Week 144 | |
| mg/dL | Change† | mg/dL | Change† | |
| Total Cholesterol (fasted) | 162 [N=647] | +31 [N=647] | 165 [N=627] | +14 [N=627] |
| Triglycerides (fasted) | 111 [N=647] | +29 [N=647] | 115 [N=627] | +17 [N=627] |
| LDL-cholesterol (fasted) | 103 [N=647] | +20 [N=643] | 107 [N=628] | +8 [N=628] |
| HDL-cholesterol (fasted) | 47 [N=647] | +7 [N=647] | 46 [N=627] | +3 [N=627] |
| Total Cholesterol to HDL ratio | 3.7 [N=647] | 0.2 [N=647] | 3.8 [N=627] | 0.1 [N=627] |
Clinical Trials in Pediatric Subjects:
Safety in Pediatric Patients
The safety of GENVOYA in HIV-1 infected pediatric subjects was evaluated in treatment-naïve subjects between the ages of 12 to less than 18 years and weighing at least 35 kg (N=50) through Week 48 (cohort 1), and in virologically-suppressed subjects between the ages of 6 to less than 12 years and weighing at least 25 kg (N=23) through Week 24 (cohort 2) in an open-label clinical trial (Study 106) [see Clinical Studies (14.5)]. With the exception of a decrease in the mean CD4+ cell count observed in cohort 2 of Study 106, the safety profile in pediatric subjects who received treatment with GENVOYA was similar to that in adults. One 13-year-old female subject developed unexplained uveitis while receiving GENVOYA that resolved and did not require discontinuation of GENVOYA.
Bone Mineral Density Effects
Cohort 1: Treatment-naïve adolescents (12 to less than 18 years; at least 35 kg)
Among the subjects in cohort 1 receiving GENVOYA, mean BMD increased from baseline to Week 48, + 4.2% at the lumbar spine and + 1.3% for the total body less head (TBLH). Mean changes from baseline BMD Z-scores were −0.07 for lumbar spine and −0.20 for TBLH at Week 48. One GENVOYA subject had significant (at least 4%) lumbar spine BMD loss at Week 48.
Cohort 2: Virologically-suppressed children (6 to less than 12 years; at least 25 kg)
Among the subjects in cohort 2 receiving GENVOYA, mean BMD increased from baseline to Week 24, +2.9% at the lumbar spine and +1.7% for TBLH. Mean changes from baseline BMD Z-scores were -0.06 for lumbar spine and -0.18 for TBLH at Week 24. Two GENVOYA subjects had significant (at least 4%) lumbar spine BMD loss at Week 24.
Change from Baseline in CD4+ cell counts
Cohort 2: Virologically-suppressed children (6 to less than 12 years; at least 25 kg)
Cohort 2 of Study 106 evaluated pediatric subjects (N=23) who were virologically-suppressed and who switched from their antiretroviral regimen to GENVOYA. Although all subjects had HIV-1 RNA < 50 copies/mL, there was a decrease from baseline in CD4+ cell count at Week 24. The mean baseline and mean change from baseline in CD4+ cell count and in CD4% from Week 2 to Week 24 are presented in Table 4. All subjects maintained their CD4+ cell counts above 400 cells/mm3 [see Pediatric Use (8.4) and Clinical Studies (14.5)].
| Baseline | Mean Change from Baseline | ||||
|---|---|---|---|---|---|
| Week 2 | Week 4 | Week 12 | Week 24 | ||
|
|||||
| CD4+ Cell Count (cells/mm3) | 966 (201.7)* | -162 | -125 | -162 | -150 |
| CD4% | 40 (5.3)* | +0.5% | -0.1% | -0.8% | -1.5% |
6.2 Postmarketing Experience
The following events have been identified during post approval use of products containing TAF, including GENVOYA. Because these events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
7 DRUG INTERACTIONS
7.1 Not Recommended with Other Antiretroviral Medications
GENVOYA is a complete regimen for the treatment of HIV-1 infection; therefore, coadministration of GENVOYA with other antiretroviral medications for treatment of HIV-1 infection should be avoided. Complete information regarding potential drug-drug interactions with other antiretroviral medications is not provided [see Contraindications (4), Warnings and Precautions (5.2) and Clinical Pharmacology (12.3)].
7.2 Potential for GENVOYA to Affect Other Drugs
Cobicistat, a component of GENVOYA, is an inhibitor of CYP3A and CYP2D6 and an inhibitor of the following transporters: P-glycoprotein (P-gp), BCRP, OATP1B1 and OATP1B3. Thus, coadministration of GENVOYA with drugs that are primarily metabolized by CYP3A or CYP2D6, or are substrates of P-gp, BCRP, OATP1B1 or OATP1B3 may result in increased plasma concentrations of such drugs (see Table 5). Elvitegravir is a modest inducer of CYP2C9 and may decrease the plasma concentrations of CYP2C9 substrates. TAF is not an inhibitor of CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, or UGT1A1. TAF is a weak inhibitor of CYP3A in vitro. TAF is not an inhibitor or inducer of CYP3A in vivo.
7.3 Potential for Other Drugs to Affect One or More Components of GENVOYA
Elvitegravir and cobicistat, components of GENVOYA, are metabolized by CYP3A. Cobicistat is also metabolized, to a minor extent, by CYP2D6.
Drugs that induce CYP3A activity are expected to increase the clearance of elvitegravir and cobicistat, resulting in decreased plasma concentration of cobicistat, elvitegravir, and TAF, which may lead to loss of therapeutic effect of GENVOYA and development of resistance (see Table 5).
Coadministration of GENVOYA with other drugs that inhibit CYP3A may decrease the clearance and increase the plasma concentration of cobicistat (see Table 5).
TAF, a component of GENVOYA, is a substrate of P-gp, BCRP, OATP1B1 and OATP1B3. Drugs that inhibit P-gp and/or BCRP, such as cobicistat, may increase the absorption of TAF (see Table 13). However, when TAF is administered as a component of GENVOYA, its availability is increased by cobicistat and a further increase of TAF concentrations is not expected upon coadministration of an additional P-gp and/or BCRP inhibitor. Drugs that induce P-gp activity are expected to decrease the absorption of TAF, resulting in decreased plasma concentration of TAF.
7.4 Drugs Affecting Renal Function
Because emtricitabine and tenofovir are primarily excreted by the kidneys by a combination of glomerular filtration and active tubular secretion, coadministration of GENVOYA with drugs that reduce renal function or compete for active tubular secretion may increase concentrations of emtricitabine, tenofovir, and other renally eliminated drugs and this may increase the risk of adverse reactions. Some examples of drugs that are eliminated by active tubular secretion include, but are not limited to, acyclovir, cidofovir, ganciclovir, valacyclovir, valganciclovir, aminoglycosides (e.g., gentamicin), and high-dose or multiple NSAIDs [see Warnings and Precautions (5.4)].
7.5 Established and Other Potentially Significant Interactions
Table 5 provides a listing of established or potentially clinically significant drug interactions [see Contraindications (4)]. The drug interactions described are based on studies conducted with either GENVOYA, the components of GENVOYA (elvitegravir, cobicistat, emtricitabine, and tenofovir alafenamide) as individual agents and/or in combination, or are predicted drug interactions that may occur with GENVOYA [for magnitude of interaction, see Clinical Pharmacology (12.3)]. The table includes potentially significant interactions but is not all inclusive.
| Concomitant Drug Class: Drug Name | Effect on Concentration† | Clinical Comment |
|---|---|---|
| Acid Reducing Agents:
antacids‡ e.g., aluminum and magnesium hydroxide | ↓ elvitegravir | Separate GENVOYA and antacid administration by at least 2 hours. |
| Alpha 1-adrenoreceptor antagonist:
alfuzosin | ↑ alfuzosin | Coadministration with alfuzosin is contraindicated due to potential for serious and/or life-threatening reactions such as hypotension. |
| Antiarrhythmics:
e.g., amiodarone bepridil digoxin‡ disopyramide flecainide systemic lidocaine mexiletine propafenone quinidine | ↑ antiarrhythmics ↑ digoxin | Caution is warranted and therapeutic concentration monitoring, if available, is recommended for antiarrhythmics when coadministered with GENVOYA. |
| Antibacterials:
clarithromycin telithromycin | ↑ clarithromycin ↑ telithromycin ↑ cobicistat | Patients with CLcr greater than or equal to 60 mL/minute:
No dosage adjustment of clarithromycin is required. Patients with CLcr between 50 mL/minute and 60 mL/minute: The dosage of clarithromycin should be reduced by 50%. |
| Anticoagulants:
Direct Oral Anticoagulants (DOACs) apixaban rivaroxaban betrixaban dabigatran edoxaban | ↑ apixaban | Due to potentially increased bleeding risk, dosing recommendations for coadministration with GENVOYA depends on the apixaban dose. Refer to apixaban dosing instructions for coadministration with strong CYP3A and P-gp inhibitors in apixaban prescribing information. |
| ↑ rivaroxaban ↑ betrixaban ↑ dabigatran ↑ edoxaban | Coadministration of rivaroxaban with GENVOYA is not recommended because it may lead to an increased bleeding risk. Due to potentially increased bleeding risk, dosing recommendations for coadministration of betrixaban, dabigatran, or edoxaban with a P-gp inhibitor such as GENVOYA depends on DOAC indication and renal function. Refer to DOAC dosing instructions for coadministration with P-gp inhibitors in DOAC prescribing information. |
|
| warfarin | Effect on warfarin unknown | Monitor the international normalized ratio (INR) upon coadministration of warfarin with GENVOYA. |
| Anticonvulsants:
carbamazepine‡ phenobarbital phenytoin | ↓ elvitegravir ↓ cobicistat ↓ TAF | Coadministration with carbamazepine, phenobarbital, or phenytoin is contraindicated due to potential for loss of therapeutic effect and development of resistance. |
| oxcarbazepine | Alternative anticonvulsants should be considered when GENVOYA is administered with oxcarbazepine. | |
| ethosuximide | ↑ ethosuximide | Clinical monitoring is recommended upon coadministration of ethosuximide with GENVOYA. |
| Antidepressants:
Selective Serotonin Reuptake Inhibitors (SSRIs) e.g., paroxetine Tricyclic Antidepressants (TCAs) e.g., amitriptyline desipramine‡ imipramine nortriptyline bupropion trazodone | ↑ SSRIs (except sertraline) ↑ TCAs ↑ trazodone | Careful dosage titration of the antidepressant and monitoring for antidepressant response are recommended when coadministered with GENVOYA. |
| Antifungals:
itraconazole ketoconazole‡ voriconazole | ↑ elvitegravir ↑ cobicistat ↑ itraconazole ↑ ketoconazole ↑ voriconazole | When administering with GENVOYA, the maximum daily dosage of ketoconazole or itraconazole should not exceed 200 mg per day. An assessment of benefit/risk ratio is recommended to justify use of voriconazole with GENVOYA. |
| Anti-gout:
colchicine | ↑ colchicine | GENVOYA is not recommended to be coadministered with colchicine to patients with renal or hepatic impairment. Treatment of gout-flares – coadministration of colchicine in patients receiving GENVOYA: 0.6 mg (1 tablet) × 1 dose, followed by 0.3 mg (half tablet) 1 hour later. Treatment course to be repeated no earlier than 3 days. Prophylaxis of gout-flares – coadministration of colchicine in patients receiving GENVOYA: If the original regimen was 0.6 mg twice a day, the regimen should be adjusted to 0.3 mg once a day. If the original regimen was 0.6 mg once a day, the regimen should be adjusted to 0.3 mg once every other day. Treatment of familial Mediterranean fever – coadministration of colchicine in patients receiving GENVOYA: Maximum daily dosage of 0.6 mg (may be given as 0.3 mg twice a day). |
| Antimycobacterial:
rifampin | ↓ elvitegravir ↓ cobicistat ↓ TAF | Coadministration with rifampin is contraindicated due to potential for loss of therapeutic effect and development of resistance . |
| rifabutin‡
rifapentine | Coadministration of GENVOYA with rifabutin or rifapentine is not recommended. | |
| Antipsychotics: | ||
| lurasidone | ↑ lurasidone | Coadministration with lurasidone is contraindicated due to potential for serious and/or life-threatening reactions. |
| pimozide | ↑ pimozide | Coadministration with pimozide is contraindicated due to potential for serious and/or life-threatening reactions such as cardiac arrhythmias. |
| quetiapine | ↑ quetiapine | Initiation of GENVOYA in patients taking quetiapine:
Consider alternative antiretroviral therapy to avoid increases in quetiapine exposure. If coadministration is necessary, reduce the quetiapine dose to 1/6 of the current dose and monitor for quetiapine-associated adverse reactions. Refer to the quetiapine prescribing information for recommendations on adverse reaction monitoring. Initiation of quetiapine in patients taking GENVOYA: Refer to the quetiapine prescribing information for initial dosing and titration of quetiapine. |
| Other antipsychotics e.g., perphenazine risperidone thioridazine | ↑ antipsychotic | A decrease in dose of the antipsychotics that are metabolized by CYP3A or CYP2D6 may be needed when coadministered with GENVOYA. |
| Beta-Blockers:
e.g., metoprolol timolol | ↑ beta-blockers | Clinical monitoring is recommended and a dosage decrease of the beta blocker may be necessary when these agents are coadministered with GENVOYA. |
| Calcium Channel Blockers:
e.g., amlodipine diltiazem felodipine nicardipine nifedipine verapamil | ↑ calcium channel blockers | Caution is warranted and clinical monitoring is recommended upon coadministration of calcium channel blockers with GENVOYA. |
| Corticosteroids (all routes excluding cutaneous): e.g., betamethasone budesonide ciclesonide dexamethasone fluticasone methylprednisolone mometasone prednisone triamcinolone | ↓ elvitegravir ↓ cobicistat ↑ corticosteroids | Coadministration with oral dexamethasone or other systemic corticosteroids that induce CYP3A may result in loss of therapeutic effect and development of resistance to elvitegravir. Consider alternative corticosteroids. Coadministration with corticosteroids whose exposures are significantly increased by strong CYP3A inhibitors can increase the risk for Cushing's syndrome and adrenal suppression. Alternative corticosteroids including beclomethasone and prednisolone (whose PK and/or PD are less affected by strong CYP3A inhibitors relative to other studied steroids) should be considered, particularly for long-term use. |
| Endothelin Receptor Antagonists:
bosentan | ↑ bosentan | Coadministration of bosentan in patients on GENVOYA:
In patients who have been receiving GENVOYA for at least 10 days, start bosentan at 62.5 mg once daily or every other day based upon individual tolerability. Coadministration of GENVOYA in patients on bosentan: Discontinue use of bosentan at least 36 hours prior to initiation of GENVOYA. After at least 10 days following the initiation of GENVOYA, resume bosentan at 62.5 mg once daily or every other day based upon individual tolerability. |
| Ergot Derivatives:
dihydroergotamine ergotamine methylergonovine | ↑ ergot derivatives | Coadministration is contraindicated due to potential for serious and/or life-threatening reactions such as acute ergot toxicity characterized by peripheral vasospasm and ischemia of the extremities and other tissues [see Contraindications (4)]. |
| GI Motility Agent:
cisapride | ↑ cisapride | Coadministration is contraindicated due to potential for serious and/or life-threatening reactions such as cardiac arrhythmias. |
| Herbal Products:
St. John's wort (Hypericum perforatum) | ↓ elvitegravir ↓ cobicistat ↓ TAF | Coadministration is contraindicated due to potential for loss of therapeutic effect and development of resistance. |
| Hormonal Contraceptives:
drospirenone/ethinyl estradiol‡ levonorgestrel norgestimate/ethinyl estradiol | ↑ drospirenone ↑ norgestimate ↑ levonorgestrel ↓ ethinyl estradiol | Additional or alternative non-hormonal forms of contraception should be considered when estrogen based contraceptives are coadministered with GENVOYA. Plasma concentrations of drospirenone may be increased when coadministered with cobicistat-containing products. Clinical monitoring is recommended due to the potential for hyperkalemia. The effects of increases in the concentration of the progestational component norgestimate are not fully known and can include increased risk of insulin resistance, dyslipidemia, acne, and venous thrombosis. The potential risks and benefits associated with coadministration of norgestimate/ethinyl estradiol with GENVOYA should be considered, particularly in patients who have risk factors for these events. The effect of GENVOYA on other hormonal contraceptives (e.g., contraceptive patch, contraceptive vaginal ring, or injectable contraceptives) or oral contraceptives containing progestogens other than drospirenone, levonorgestrel, or norgestimate has not been studied; therefore, alternative (non-hormonal) methods of contraception can be considered. |
| Immuno-suppressants:
e.g., cyclosporine (CsA) sirolimus tacrolimus | ↑ immuno-suppressants ↑ elvitegravir (with CsA) ↑ cobicistat (with CsA) | Therapeutic monitoring of the immunosuppressive agents is recommended upon coadministration with GENVOYA. Monitor for adverse events associated with GENVOYA when coadministered with cyclosporine. |
| Lipid-modifying Agents: | ||
| HMG-CoA Reductase Inhibitors:
lovastatin simvastatin | ↑ lovastatin ↑ simvastatin | Coadministration with lovastatin or simvastatin is contraindicated due to potential for serious reactions such as myopathy including rhabdomyolysis. |
| atorvastatin | ↑ atorvastatin | Initiate atorvastatin with the lowest starting dose of atorvastatin and titrate carefully while monitoring for safety (e.g., myopathy). Do not exceed a dosage of atorvastatin 20 mg daily. |
| Other Lipid-modifying Agents:
lomitapide | ↑ lomitapide | Coadministration with lomitapide is contraindicated due to potential for markedly increased transaminases. |
| Narcotic Analgesics:
buprenorphine/ naloxone‡ | ↑ buprenorphine ↑ norbuprenorphine ↓ naloxone | No dosage adjustment of buprenorphine/naloxone is required upon coadministration with GENVOYA. Patients should be closely monitored for sedation and cognitive effects. |
| fentanyl | ↑ fentanyl | Careful monitoring of therapeutic and adverse effects of fentanyl (including potentially fatal respiratory depression) is recommended with coadministration. |
| tramadol | ↑ tramadol | A dose decrease may be needed for tramadol with concomitant use. |
| Inhaled Beta Agonist:
salmeterol | ↑ salmeterol | Coadministration of salmeterol and GENVOYA is not recommended. Coadministration of salmeterol with GENVOYA may result in increased risk of cardiovascular adverse events associated with salmeterol, including QT prolongation, palpitations, and sinus tachycardia. |
| Phosphodiesterase-5 (PDE5) Inhibitors:
sildenafil tadalafil vardenafil | ↑ PDE5 inhibitors | Use of PDE-5 inhibitors for pulmonary arterial hypertension (PAH):
Coadministration of sildenafil with GENVOYA is contraindicated when used for treatment of PAH, due to potential for PDE-5 inhibitor associated adverse reactions, including hypotension, syncope, visual disturbances, and priapism. The following dose adjustments are recommended for the use of tadalafil with GENVOYA: Coadministration of tadalafil in patients on GENVOYA: In patients receiving GENVOYA for at least 1 week, start tadalafil at 20 mg once daily. Increase tadalafil dose to 40 mg once daily based upon individual tolerability. Coadministration of GENVOYA in patients on tadalafil: Avoid use of tadalafil during the initiation of GENVOYA. Stop tadalafil at least 24 hours prior to starting GENVOYA. After at least one week following initiation of GENVOYA, resume tadalafil at 20 mg once daily. Increase tadalafil dose to 40 mg once daily based upon individual tolerability. Use of PDE-5 inhibitors for erectile dysfunction: Sildenafil at a single dose not exceeding 25 mg in 48 hours, vardenafil at a single dose not exceeding 2.5 mg in 72 hours, or tadalafil at a single dose not exceeding 10 mg in 72 hours can be used with increased monitoring for PDE-5 inhibitor associated with adverse events. |
| Sedative/hypnotics:
midazolam (oral) triazolam | ↑ midazolam ↑ triazolam | Coadministration with triazolam or orally administered midazolam is contraindicated due to potential for serious and/or life-threatening reactions such as prolonged or increased sedation or respiratory depression. |
| Other benzodiazepines: e.g., parenterally administered midazolam clorazepate diazepam estazolam flurazepam | ↑ sedatives/hypnotics | Triazolam and orally administered midazolam are extensively metabolized by CYP3A. Coadministration of triazolam or orally administered midazolam with GENVOYA may cause large increases in the concentrations of these benzodiazepines. Coadministration of parenteral midazolam with GENVOYA should be done in a setting that ensures close clinical monitoring and appropriate medical management in case of respiratory depression and/or prolonged sedation. Dosage reduction for midazolam should be considered, especially if more than a single dose of midazolam is administered. |
| buspirone zolpidem | With other sedative/hypnotics, dose reduction may be necessary and clinical monitoring is recommended. | |
7.6 Drugs without Clinically Significant Interactions with GENVOYA
Based on drug interaction studies conducted with the components of GENVOYA, no clinically significant drug interactions have been observed when GENVOYA is combined with the following drugs: famciclovir, famotidine, ledipasvir, methadone, omeprazole, sertraline, sofosbuvir, velpatasvir, and voxilaprevir.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in individuals exposed to GENVOYA during pregnancy. Healthcare providers are encouraged to register patients by calling the Antiretroviral Pregnancy Registry (APR) at 1-800-258-4263.
Risk Summary
GENVOYA is not recommended during pregnancy [see Dosage and Administration (2.5)]. A literature report evaluating the pharmacokinetics of antiretrovirals during pregnancy demonstrated substantially lower exposures of elvitegravir and cobicistat in the second and third trimesters (see Data).
Prospective pregnancy data from the APR are not sufficient to adequately assess the risk of birth defects or miscarriage. However, elvitegravir, cobicistat, emtricitabine, and TAF use during pregnancy have been evaluated in a limited number of individuals as reported to the APR. Available data from the APR show no increase in the overall risk of major birth defects for emtricitabine or cobicistat compared with the background rate for major birth defects of 2.7% in a U.S. reference population of the Metropolitan Atlanta Congenital Defects Program (MACDP). The number of exposures to TAF and elvitegravir are insufficient to make a risk assessment compared to a reference population (see Data). The rate of miscarriage is not reported in the APR. In the U.S. general population, the estimated background risk of miscarriage in clinically recognized pregnancies is 15-20%.
In animal studies, no adverse developmental effects were observed when the components of GENVOYA were administered separately during the period of organogenesis at exposures up to 23 and 0.2 times (rat and rabbits, respectively: elvitegravir), 1.6 and 3.8 times (rats and rabbits, respectively: cobicistat), 60 and 108 times (mice and rabbits, respectively; emtricitabine) and equal to and 53 times (rats and rabbits, respectively; TAF) the exposure at the recommended daily dosage of these components in GENVOYA (see Data). Likewise, no adverse developmental effects were seen when elvitegravir or cobicistat was administered to rats through lactation at exposures up to 18 times or 1.2 times, respectively, the human exposure at the recommended therapeutic dose, and when emtricitabine was administered to mice through lactation at exposures up to approximately 60 times the exposure at the recommended daily dose. No adverse effects were observed in the offspring when TDF was administered through lactation at tenofovir exposures of approximately 14 times the exposure at the recommended daily dosage of GENVOYA.
Data
Human Data
A prospective study, reported in the literature, enrolled 30 pregnant women living with HIV who were receiving elvitegravir and cobicistat-based regimens in the second or third trimesters of pregnancy and through 6 to 12 weeks postpartum to evaluate the pharmacokinetics (PK) of antiretrovirals during pregnancy. Twenty-eight women completed the study through the postpartum period. Paired pregnancy/postpartum PK data were available from 14 and 24 women for the second and third trimesters, respectively. Exposures of elvitegravir and cobicistat were substantially lower during the second and third trimesters compared to postpartum. The proportion of pregnant women who were virologically suppressed was 77% in the second trimester, 92% in the third trimester, and 76% postpartum. No correlation was observed between viral suppression and elvitegravir exposure. HIV status was also assessed for infants: 25 were uninfected, 2 had indeterminate status, and no information was available for 3 infants.
Prospective reports from the APR of overall major birth defects in pregnancies exposed to the components of GENVOYA are compared with a U.S. background major birth defect rate. Methodological limitations of the APR include the use of MACDP as the external comparator group. Limitations of using an external comparator include differences in methodology and populations, as well as confounding due to the underlying disease.
Elvitegravir:
The APR has received prospective reports of 5 birth defects among 180 first trimester exposures to elvitegravir-containing regimens during pregnancy resulting in live births. No birth defects were reported among 52 exposures during the second/third trimester. The number of exposures is insufficient to make a risk assessment compared to a reference population.
Cobicistat:
Based on prospective reports to the APR of 204 first trimester exposures to cobicistat-containing regimens during pregnancy, there was no increase in overall major birth defects with cobicistat compared with the background birth defect rate of 2.7% in the U.S. reference population of the MACDP. The prevalence of birth defects in live births was 2.5% (95% CI: 0.8% to 5.6%) with first trimester exposure to cobicistat-containing regimens. The 58 second/third trimester cobicistat exposures reported to the APR are insufficient to make a risk assessment.
Emtricitabine (FTC):
Based on prospective reports to the APR of exposures to emtricitabine-containing regimens during pregnancy resulting in live births (including over 2,700 exposed in the first trimester and over 1,200 exposed in the second/third trimester), there was no increase in overall major birth defects with FTC compared with the background birth defect rate of 2.7% in the U.S. reference population of the MACDP. The prevalence of birth defects in live births was 2.4% (95% CI: 1.9% to 3.1%) with first trimester exposure to FTC-containing regimens and 2.3% (95% CI: 1.5% to 3.3%) with second/third trimester exposure to emtricitabine-containing regimens.
Tenofovir Alafenamide (TAF):
The APR has received prospective reports of 3 birth defects among 56 first trimester exposures to TAF-containing regimens during pregnancy resulting in live births. No birth defects were reported among 29 exposures during the second/third trimester. The number of exposures is insufficient to make a risk assessment compared to a reference population.
Animal Data
Elvitegravir:
Elvitegravir was administered orally to pregnant rats (0, 300, 1000, and 2000 mg/kg/day) and rabbits (0, 50, 150, and 450 mg/kg/day) through organogenesis (on gestation days 7 through 17 and days 7 through 19, respectively). No significant toxicological effects were observed in embryo-fetal toxicity studies performed with elvitegravir in rats at exposures (AUC) approximately 23 times and in rabbits at approximately 0.2 times the human exposures at the recommended daily dose. In a pre/postnatal developmental study, elvitegravir was administered orally to rats at doses of 0, 300, 1000, and 2000 mg/kg from gestation day 7 to day 20 of lactation. At doses of 2000 mg/kg/day of elvitegravir, neither maternal nor developmental toxicity was noted. Systemic exposures (AUC) at this dose were 18 times the human exposures at the recommended daily dose.
Cobicistat:
Cobicistat was administered orally to pregnant rats at doses of 0, 25, 50, 125 mg/kg/day on gestation day 6 to 17. Increases in post-implantation loss and decreased fetal weights were observed at a maternal toxic dose of 125 mg/kg/day. No malformations were noted at doses up to 125 mg/kg/day. Systemic exposures (AUC) at 50 mg/kg/day in pregnant females were 1.6 times higher than human exposures at the recommended daily dose.
In pregnant rabbits, cobicistat was administered orally at doses of 0, 20, 50, and 100 mg/kg/day during gestation days 7 to 20. No maternal or embryo/fetal effects were noted at the highest dose of 100 mg/kg/day. Systemic exposures (AUC) at 100 mg/kg/day were 3.8 times higher than human exposures at the recommended daily dose.
In a pre/postnatal developmental study in rats, cobicistat was administered orally at doses of 0, 10, 30, and 75 mg/kg from gestation day 6 to postnatal day 20, 21, or 22. At doses of 75 mg/kg/day of cobicistat, neither maternal nor developmental toxicity was noted. Systemic exposures (AUC) at this dose were 1.2 times the human exposures at the recommended daily dose.
Emtricitabine:
Emtricitabine was administered orally to pregnant mice (250, 500, or 1000 mg/kg/day) and rabbits (100, 300, or 1000 mg/kg/day) through organogenesis (on gestation days 6 through 15, and 7 through 19, respectively). No significant toxicological effects were observed in embryo-fetal toxicity studies performed with emtricitabine in mice at exposures (AUC) approximately 60 times higher and in rabbits at approximately 108 times higher than human exposures at the recommended daily dose.
In a pre/postnatal development study with emtricitabine, mice were administered doses up to 1000 mg/kg/day; no significant adverse effects directly related to drug were observed in the offspring exposed daily from before birth (in utero) through sexual maturity at daily exposures (AUC) of approximately 60 times higher than human exposures at the recommended daily dose.
Tenofovir Alafenamide (TAF):
TAF was administered orally to pregnant rats (25, 100, or 250 mg/kg/day) and rabbits (10, 30, or 100 mg/kg/day) through organogenesis (on gestation days 6 through 17, and 7 through 20, respectively). No adverse embryo-fetal effects were observed in rats and rabbits at TAF exposures similar to (rats) and approximately 53 (rabbits) times higher than the exposure in humans at the recommended daily dose of GENVOYA. TAF is rapidly converted to tenofovir; the observed tenofovir exposure in rats and rabbits were 59 (rats) and 93 (rabbits) times higher than human tenofovir exposures at the recommended daily doses. Since TAF is rapidly converted to tenofovir and lower tenofovir exposures in rats and mice were observed after TAF administration compared to TDF administration, a pre/postnatal development study in rats was conducted only with TDF. Doses up to 600 mg/kg/day were administered through lactation; no adverse effects were observed in the offspring on gestation day 7 [and lactation day 20] at tenofovir exposures of approximately 14 [21] times higher than the exposures in humans at the recommended daily dose of GENVOYA.
8.2 Lactation
Risk Summary
The Centers for Disease Control and Prevention recommend that HIV-infected mothers not breastfeed their infants to avoid risking postnatal transmission of HIV.
Based on published data, emtricitabine has been shown to be present in human breast milk; it is unknown if elvitegravir, cobicistat, and TAF are present in human breast milk. Elvitegravir and cobicistat are present in rat milk, and tenofovir has been shown to be present in the milk of lactating rats and rhesus monkeys after administration of TDF [see Data]. It is unknown if TAF is present in animal milk.
It is not known if GENVOYA affects milk production or has effects on the breastfed child. Because of the potential for 1) HIV transmission (in HIV-negative infants); 2) developing viral resistance (in HIV-positive infants); and 3) adverse reactions in a breastfed infant similar to those seen in adults, instruct mothers not to breastfeed if they are receiving GENVOYA.
Data
Animal Data
Elvitegravir: During the pre/postnatal developmental toxicology study at doses up to 2000 mg/kg/day, a mean elvitegravir milk to plasma ratio of 0.1 was measured 30 minutes after administration to rats on lactation day 14.
Cobicistat: During the pre/postnatal developmental toxicology study at doses up to 75 mg/kg/day, mean cobicistat milk to plasma ratio of up to 1.9 was measured 2 hours after administration to rats on lactation day 10.
Tenofovir Alafenamide: Studies in rats and monkeys have demonstrated that tenofovir is secreted in milk. During the pre/postnatal developmental toxicology study, tenofovir was excreted into the milk of lactating rats following oral administration of TDF (up to 600 mg/kg/day) at up to approximately 24% of the median plasma concentration in the highest dosed animals at lactation day 11. Tenofovir was excreted into the milk of lactating rhesus monkeys, following a single subcutaneous (30 mg/kg) dose of tenofovir, at concentrations up to approximately 4% of plasma concentration resulting in exposure (AUC) of approximately 20% of plasma exposure.
8.4 Pediatric Use
The safety and effectiveness of GENVOYA for the treatment of HIV-1 infection was established in pediatric patients with body weight greater than or equal to 25 kg [see Indications and Usage (1) and Dosage and Administration (2.2)].
Use of GENVOYA in pediatric patients between the ages of 12 to less than 18 years and weighing at least 35 kg is supported by studies in adults and by a study in antiretroviral treatment-naïve HIV-1 infected pediatric subjects ages 12 to less than 18 years and weighing at least 35 kg (cohort 1 of Study 106, N=50). The safety and efficacy of GENVOYA in these pediatric subjects was similar to that in adults [see Adverse Reactions (6.1), Clinical Pharmacology (12.3), and Clinical Studies (14.5)].
Use of GENVOYA in pediatric patients weighing at least 25 kg is supported by studies in adults and by an open-label trial in virologically-suppressed pediatric subjects ages 6 to less than 12 years and weighing at least 25 kg, in which subjects were switched from their antiretroviral regimen to GENVOYA (cohort 2 of Study 106, N=23). The safety in these subjects through 24 weeks was similar to that in antiretroviral treatment-naïve adults with the exception of a decrease in mean change from baseline in CD4+ cell count [see Adverse Reactions (6.1), Clinical Pharmacology (12.3), and Clinical Studies (14.5)].
Safety and effectiveness of GENVOYA in pediatric patients less than 25 kg have not been established.
8.5 Geriatric Use
Clinical trials of GENVOYA included 97 subjects (80 receiving GENVOYA) aged 65 years and over. No differences in safety or efficacy have been observed between elderly subjects and adults between 18 and less than 65 years of age.
8.6 Renal Impairment
The pharmacokinetics, safety, and virologic and immunologic responses of GENVOYA in HIV-1 infected adult subjects with renal impairment (estimated creatinine clearance between 30 and 69 mL per minute by Cockcroft-Gault method) were evaluated in 248 subjects in an open-label trial, Study 112.
The pharmacokinetics, safety, virologic and immunologic responses of GENVOYA in HIV-1 infected adult subjects with ESRD (estimated creatinine clearance of less than 15 mL per minute by Cockcroft-Gault method) receiving chronic hemodialysis were evaluated in 55 subjects in an open-label trial, Study 1825 [see Adverse Reactions (6.1) and Clinical Studies (14.4)].
No dosage adjustment of GENVOYA is recommended in patients with estimated creatinine clearance greater than or equal to 30 mL per minute, or in adult patients with ESRD (estimated creatinine clearance below 15 mL per minute) who are receiving chronic hemodialysis. On days of hemodialysis, administer GENVOYA after completion of hemodialysis treatment [see Dosage and Administration (2.2)].
GENVOYA is not recommended in patients with severe renal impairment (estimated creatinine clearance of 15 to below 30 mL per minute), or in patients with ESRD who are not receiving chronic hemodialysis, as the safety of GENVOYA has not been established in these populations [see Dosage and Administration (2.3), Warnings and Precautions (5.4) and Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
No dosage adjustment of GENVOYA is required in patients with mild (Child-Pugh Class A) or moderate (Child-Pugh Class B) hepatic impairment. GENVOYA has not been studied in patients with severe hepatic impairment (Child-Pugh Class C). Therefore, GENVOYA is not recommended for use in patients with severe hepatic impairment [see Dosage and Administration (2.4) and Clinical Pharmacology (12.3)].
10 OVERDOSAGE
No data are available on overdose of GENVOYA in patients. If overdose occurs, monitor the patient for evidence of toxicity. Treatment of overdose with GENVOYA consists of general supportive measures including monitoring of vital signs as well as observation of the clinical status of the patient.
Elvitegravir: Limited clinical experience is available at doses higher than the recommended dose of elvitegravir in GENVOYA. In one study, elvitegravir (administered with the CYP3A inhibitor cobicistat) equivalent to 2 times the therapeutic dose of 150 mg once daily for 10 days was administered to 42 healthy subjects. No severe adverse reactions were reported. The effects of higher doses are not known. As elvitegravir is highly bound to plasma proteins, it is unlikely that it will be significantly removed by hemodialysis or peritoneal dialysis.
Cobicistat: Limited clinical experience is available at doses higher than the recommended dose of cobicistat in GENVOYA. In two studies, a single dose of cobicistat 400 mg (2.7 times the dose in GENVOYA) was administered to a total of 60 healthy subjects. No severe adverse reactions were reported. The effects of higher doses are not known. As cobicistat is highly bound to plasma proteins, it is unlikely that it will be significantly removed by hemodialysis or peritoneal dialysis.
Emtricitabine: Limited clinical experience is available at doses higher than the recommended dose of emtricitabine in GENVOYA. In one clinical pharmacology study, single doses of emtricitabine 1200 mg (6 times the dose in GENVOYA) were administered to 11 subjects. No severe adverse reactions were reported. The effects of higher doses are not known.
Hemodialysis treatment removes approximately 30% of the emtricitabine dose over a 3-hour dialysis period starting within 1.5 hours of emtricitabine dosing (blood flow rate of 400 mL per minute and a dialysate flow rate of 600 mL per minute). It is not known whether emtricitabine can be removed by peritoneal dialysis.
Tenofovir alafenamide (TAF): Limited clinical experience is available at doses higher than the recommended dose of TAF in GENVOYA. A single dose of 125 mg TAF (12.5 times the dose in GENVOYA) was administered to 48 healthy subjects; no serious adverse reactions were reported. The effects of higher doses are unknown. Tenofovir is efficiently removed by hemodialysis with an extraction coefficient of approximately 54%.
11 DESCRIPTION
GENVOYA (elvitegravir, cobicistat, emtricitabine, and tenofovir alafenamide) is a fixed-dose combination tablet containing elvitegravir, cobicistat, emtricitabine, and tenofovir alafenamide for oral administration.
- Elvitegravir is an HIV-1 integrase strand transfer inhibitor.
- Cobicistat is a mechanism-based inhibitor of cytochrome P450 (CYP) enzymes of the CYP3A family.
- Emtricitabine, a synthetic nucleoside analog of cytidine, is an HIV nucleoside analog reverse transcriptase inhibitor (HIV NRTI).
- Tenofovir alafenamide, an HIV NRTI, is converted in vivo to tenofovir, an acyclic nucleoside phosphonate (nucleotide) analog of adenosine 5'-monophosphate.
Each tablet contains 150 mg of elvitegravir, 150 mg of cobicistat, 200 mg of emtricitabine, and 10 mg of tenofovir alafenamide (equivalent to 11.2 mg of tenofovir alafenamide fumarate). The tablets include the following inactive ingredients: croscarmellose sodium, hydroxypropyl cellulose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, silicon dioxide, and sodium lauryl sulfate. The tablets are film-coated with a coating material containing FD&C Blue No. 2/indigo carmine aluminum lake, iron oxide yellow, polyethylene glycol, polyvinyl alcohol, talc, and titanium dioxide.
Elvitegravir: The chemical name of elvitegravir is 6-(3-chloro-2-fluorobenzyl)-1-[(2S)-1-hydroxy-3-methylbutan-2-yl]-7-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid.
It has a molecular formula of C23H23ClFNO5 and a molecular weight of 447.88. It has the following structural formula:
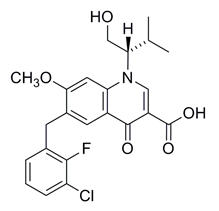
Elvitegravir is a white to pale yellow powder with a solubility of less than 0.3 micrograms per mL in water at 20 °C.
Cobicistat: The chemical name for cobicistat is 2,7,10,12-tetraazatridecanoic acid, 12-methyl-13-[2-(1-methylethyl)-4-thiazolyl]-9-[2-(4-morpholinyl)ethyl]-8,11-dioxo-3,6-bis(phenylmethyl)-, 5-thiazolylmethyl ester, (3R,6R,9S)-.
It has a molecular formula of C40H53N7O5S2 and a molecular weight of 776.02. It has the following structural formula:
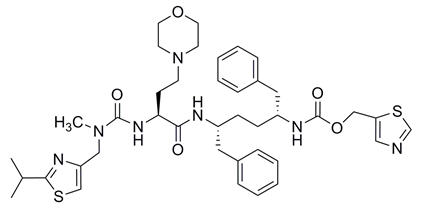
Cobicistat is adsorbed onto silicon dioxide. Cobicistat on silicon dioxide drug substance is a white to pale yellow powder with a solubility of 0.1 mg per mL in water at 20 °C.
Emtricitabine: The chemical name of emtricitabine is 4-amino-5-fluoro-1-(2R-hydroxymethyl-1,3-oxathiolan-5S-yl)-(1H)-pyrimidin-2-one. Emtricitabine is the (-)-enantiomer of a thio analog of cytidine, which differs from other cytidine analogs in that it has a fluorine in the 5 position.
It has a molecular formula of C8H10FN3O3S and a molecular weight of 247.24. It has the following structural formula:
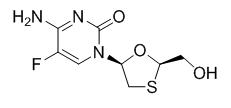
Emtricitabine is a white to off-white powder with a solubility of approximately 112 mg per mL in water at 25 °C.
Tenofovir alafenamide (TAF): The chemical name of tenofovir alafenamide fumarate drug substance is L-alanine, N-[(S)-[[(1R)-2-(6-amino-9H-purin-9-yl)-1-methylethoxy]methyl]phenoxyphosphinyl]-, 1-methylethyl ester, (2E)-2-butenedioate (2:1).
It has an empirical formula of C21H29O5N6P∙½(C4H4O4) and a formula weight of 534.5. It has the following structural formula:
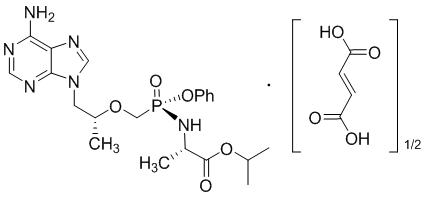
Tenofovir alafenamide fumarate is a white to off-white or tan powder with a solubility of 4.7 mg per mL in water at 20 °C.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
GENVOYA is a fixed-dose combination of antiretroviral drugs elvitegravir (plus the CYP3A inhibitor cobicistat), emtricitabine, and tenofovir alafenamide [see Microbiology (12.4)].
12.2 Pharmacodynamics
Cardiac Electrophysiology
Thorough QT studies have been conducted for elvitegravir, cobicistat, and TAF. The effect of emtricitabine or the combination regimen GENVOYA on the QT interval is not known.
Elvitegravir: In a thorough QT/QTc study in 126 healthy subjects, elvitegravir (coadministered with 100 mg ritonavir) 125 mg and 250 mg (0.83 and 1.67 times the dose in GENVOYA) did not affect the QT/QTc interval and did not prolong the PR interval.
Cobicistat: In a thorough QT/QTc study in 48 healthy subjects, a single dose of cobicistat 250 mg and 400 mg (1.67 and 2.67 times the dose in GENVOYA) did not affect the QT/QTc interval. Prolongation of the PR interval was noted in subjects receiving cobicistat. The maximum mean (95% upper confidence bound) difference in PR from placebo after baseline-correction was 9.5 (12.1) msec for the 250 mg cobicistat dose and 20.2 (22.8) for the 400 mg cobicistat dose. Because the 150 mg cobicistat dose used in the GENVOYA fixed-dose combination tablet is lower than the lowest dose studied in the thorough QT study, it is unlikely that treatment with GENVOYA will result in clinically relevant PR prolongation.
Effects on Serum Creatinine
The effect of cobicistat on serum creatinine was investigated in a Phase 1 study in subjects with an estimated creatinine clearance of at least 80 mL per minute (N=18) and with an estimated creatinine clearance of 50 to 79 mL per minute (N=12). A statistically significant change of estimated creatinine clearance from baseline was observed after 7 days of treatment with cobicistat 150 mg among subjects with an estimated creatinine clearance of at least 80 mL per minute (−9.9 ± 13.1 mL/min) and subjects with an estimated creatinine clearance between 50 and 79 mL per minute (−11.9 ± 7.0 mL per minute). These decreases in estimated creatinine clearance were reversible after cobicistat was discontinued. The actual glomerular filtration rate, as determined by the clearance of probe drug iohexol, was not altered from baseline following treatment of cobicistat among subjects with an estimated creatinine clearance of at least 50 mL per minute, indicating cobicistat inhibits tubular secretion of creatinine, reflected as a reduction in estimated creatinine clearance without affecting the actual glomerular filtration rate.
12.3 Pharmacokinetics
Absorption, Distribution, Metabolism, and Excretion
The pharmacokinetic (PK) properties of the components of GENVOYA are provided in Table 6. The multiple dose PK parameters of elvitegravir, cobicistat, emtricitabine, TAF and its metabolite tenofovir are provided in Table 7.
| Elvitegravir | Cobicistat | Emtricitabine | TAF | |
|---|---|---|---|---|
| PBMCs = peripheral blood mononuclear cells; CES1 = carboxylesterase 1. | ||||
|
||||
| Absorption | ||||
| Tmax (h) | 4 | 3 | 3 | 1 |
| Effect of light meal (relative to fasting): AUC Ratio* | 1.34 (1.19, 1.51) | 1.03 (0.90, 1.17) | 0.95 (0.91, 1.00) | 1.15 (1.07, 1.24) |
| Effect of high fat meal (relative to fasting): AUC Ratio* | 1.87 (1.66, 2.10) | 0.83 (0.73, 0.95) | 0.96 (0.92, 1.00) | 1.18 (1.09, 1.26) |
| Distribution | ||||
| % Bound to human plasma proteins | ~99 | ~98 | <4 | ~80 |
| Source of protein binding data | Ex vivo | In vitro | In vitro | Ex vivo |
| Blood-to-plasma ratio | 0.73 | 0.5 | 0.6 | 1.0 |
| Metabolism | ||||
| Metabolism | CYP3A (major) UGT1A1/3 (minor) | CYP3A (major) CYP2D6 (minor) | Not significantly metabolized | Cathepsin A† (PBMCs) CES1 (hepatocytes) CYP3A (minimal) |
| Elimination | ||||
| Major route of elimination | Metabolism | Metabolism | Glomerular filtration and active tubular secretion | Metabolism (>80% of oral dose) |
| t1/2 (h)‡ | 12.9 | 3.5 | 10 | 0.51 |
| % Of dose excreted in urine§ | 6.7 | 8.2 | 70 | <1% |
| % Of dose excreted in feces§ | 94.8 | 86.2 | 13.7 | 31.7 |
| Parameter Mean (CV%) | Elvitegravir* | Cobicistat* | Emtricitabine* | TAF† | Tenofovir‡ |
|---|---|---|---|---|---|
| CV = Coefficient of Variation; NA = Not Applicable | |||||
|
|||||
| Cmax
(microgram per mL) | 2.1 (33.7) | 1.5 (28.4) | 2.1 (20.2) | 0.16 (51.1) | 0.02 (26.1) |
| AUCtau
(microgram∙hour per mL) | 22.8 (34.7) | 9.5 (33.9) | 11.7 (16.6) | 0.21 (71.8) | 0.29 (27.4) |
| Ctrough
(microgram per mL) | 0.29 (61.7) | 0.02 (85.2) | 0.10 (46.7) | NA | 0.01 (28.5) |
Special Populations
Geriatric Patients
Pharmacokinetics of elvitegravir, cobicistat, emtricitabine and tenofovir have not been fully evaluated in the elderly (65 years of age and older). Age does not have a clinically relevant effect on exposures of TAF up to 75 years of age [see Use in Specific Populations (8.5)].
Pediatric Patients
Mean exposures of elvitegravir, cobicistat, and TAF achieved in 24 pediatric subjects aged 12 to less than 18 years who received GENVOYA in Study 106 were decreased compared to exposures achieved in treatment-naïve adults following administration of GENVOYA, but were overall deemed acceptable based on exposure-response relationships; emtricitabine exposure in adolescents was similar to that in treatment-naïve adults (Table 8).
| Parameter Mean (CV%) | Elvitegravir | Cobicistat | Emtricitabine | TAF | Tenofovir |
|---|---|---|---|---|---|
| CV = Coefficient of Variation; NA = Not Applicable | |||||
| Cmax
(microgram per mL) | 2.2 (19.2) | 1.2 (35.0) | 2.3 (22.5) | 0.17 (64.4) | 0.02 (23.7) |
| AUCtau
(microgram∙hour per mL) | 23.8 (25.5) | 8.2†
(36.1) | 14.4 (23.9) | 0.20†
(50.0) | 0.29†
(18.8) |
| Ctrough
(microgram per mL) | 0.30 (81.0) | 0.03‡
(180.0) | 0.10†
(38.9) | NA | 0.01 (21.4) |
Exposures of the components of GENVOYA achieved in 23 pediatric subjects between the ages of 6 to less than 12 years who received GENVOYA in Study 106 were higher (20 to 80% for AUC) than exposures achieved in adults following the administration of GENVOYA; however, the increase was not considered clinically significant (Table 9) [see Use in Specific Populations (8.4)].
| Parameter Mean (CV%) | Elvitegravir | Cobicistat | Emtricitabine | TAF | Tenofovir |
|---|---|---|---|---|---|
| CV = Coefficient of Variation; NA = Not Applicable | |||||
| Cmax
(microgram per mL) | 3.1 (38.7) | 2.1 (46.7) | 3.4 (27.0) | 0.31 (61.2) | 0.03 (20.8) |
| AUCtau
(microgram∙hour per mL) | 33.8†
(57.8) | 15.9‡
(51.7) | 20.6†
(18.9) | 0.33 (44.8) | 0.44 (20.9) |
| Ctrough
(microgram per mL) | 0.37 (118.5) | 0.1 (168.7) | 0.11 (24.1) | NA | 0.02 (24.9) |
Race, Gender
No clinically significant differences in pharmacokinetics of GENVOYA have been identified based on race or gender.
Patients with Renal Impairment
The pharmacokinetics of GENVOYA in HIV-1 infected subjects with mild or moderate renal impairment (estimated creatinine clearance between 30 and 69 mL per minute by Cockcroft-Gault method), and in HIV-1 infected subjects with ESRD (estimated creatinine clearance of less than 15 mL per minute by Cockcroft-Gault method) receiving chronic hemodialysis were evaluated in subsets of virologically suppressed subjects in respective open-label trials, Study 112 and Study 1825. The pharmacokinetics of elvitegravir, cobicistat, and tenofovir alafenamide were similar among healthy subjects, subjects with mild or moderate renal impairment, and subjects with ESRD receiving chronic hemodialysis; increases in emtricitabine and tenofovir exposures in subjects with renal impairment were not considered clinically relevant (Table 10).
| AUCtau (microgram∙hour per mL) Mean (CV%) |
||||
|---|---|---|---|---|
| Estimated Creatinine Clearance* | ≥90 mL per minute (N=18)† | 60–89 mL per minute (N=11)‡ | 30–59 mL per minute (N=18)§ | <15 mL per minute (N=12)¶ |
|
||||
| Emtricitabine | 11.4 (11.9) | 17.6 (18.2) | 23.0 (23.6) | 62.9 (48.0)# |
| Tenofovir | 0.32 (14.9) | 0.46 (31.5) | 0.61 (28.4) | 8.72 (39.4)Þ |
Patients with Hepatic Impairment
Elvitegravir and Cobicistat: A study of the pharmacokinetics of elvitegravir (administered with the CYP3A inhibitor cobicistat) was performed in healthy subjects and subjects with moderate hepatic impairment (Child-Pugh Class B). No clinically relevant differences in elvitegravir or cobicistat pharmacokinetics were observed between subjects with moderate hepatic impairment and healthy subjects [see Use in Specific Populations (8.7)].
Emtricitabine: The pharmacokinetics of emtricitabine has not been studied in subjects with hepatic impairment; however, emtricitabine is not significantly metabolized by liver enzymes, so the impact of liver impairment should be limited.
Tenofovir Alafenamide (TAF): Clinically relevant changes in TAF and tenofovir pharmacokinetics were not observed in subjects with mild to moderate (Child-Pugh Class A and B) hepatic impairment [see Use in Specific Populations (8.7)].
Hepatitis B and/or Hepatitis C Virus Co-infection
Elvitegravir: Limited data from population pharmacokinetic analysis (N=24) indicated that hepatitis B and/or C virus infection had no clinically relevant effect on the exposure of elvitegravir (administered with the CYP3A inhibitor cobicistat).
Drug Interaction Studies
[see also Contraindications (4) and Drug Interactions (7)]
The drug-drug interaction studies described in Tables 11–14 were conducted with GENVOYA, elvitegravir (coadministered with cobicistat or ritonavir), cobicistat administered alone, or TAF (administered alone or coadministered with emtricitabine).
As GENVOYA should not be administered with other antiretroviral medications, information regarding drug-drug interactions with other antiretrovirals agents is not provided.
The effects of coadministered drugs on the exposure of elvitegravir, emtricitabine, and TAF are shown in Table 11, Table 12, and Table 13 respectively. The effects of GENVOYA or its components on the exposure of coadministered drugs are shown in Table 14. For information regarding clinical recommendations, see Drug Interactions (7).
| Coadministered Drug | Dose of Coadministered Drug (mg) | Elvitegravir Dose (mg) | CYP3A Inhibitor Cobicistat or Ritonavir Dose (mg) | N | Mean Ratio of Elvitegravir Pharmacokinetic Parameters (90% CI); No effect = 1.00 |
||
|---|---|---|---|---|---|---|---|
| Cmax | AUC | Cmin | |||||
|
|||||||
| Maximum strength antacid† | 20 mL single dose given 4 hours before elvitegravir | 50 single dose | Ritonavir 100 single dose | 8 | 0.95 (0.84,1.07) | 0.96 (0.88,1.04) | 1.04 (0.93,1.17) |
| 20 mL single dose given 4 hours after elvitegravir | 10 | 0.98 (0.88,1.10) | 0.98 (0.91,1.06) | 1.00 (0.90,1.11) |
|||
| 20 mL single dose given 2 hours before elvitegravir | 11 | 0.82 (0.74,0.91) | 0.85 (0.79,0.91) | 0.90 (0.82,0.99) |
|||
| 20 mL single dose given 2 hours after elvitegravir | 10 | 0.79 (0.71,0.88) | 0.80 (0.75,0.86) | 0.80 (0.73,0.89) |
|||
| Atorvastatin | 10 single dose | 150 once daily‡ | Cobicistat 150 once daily‡ | 16 | 0.91 (0.85,0.98) | 0.92 (0.87,0.98) | 0.88 (0.81,0.96) |
| Carbamazepine | 200 twice daily | 150 once daily | Cobicistat 150 once daily | 12 | 0.55 (0.49,0.61) | 0.31 (0.28,0.33) | 0.03 (0.02,0.40) |
| Famotidine | 40 once daily given 12 hours after elvitegravir | 150 once daily | Cobicistat 150 once daily | 10 | 1.02 (0.89,1.17) | 1.03 (0.95,1.13) | 1.18 (1.05,1.32) |
| 40 once daily given simultaneously with elvitegravir | 16 | 1.00 (0.92,1.10) | 1.03 (0.98,1.08) | 1.07 (0.98,1.17) |
|||
| Ketoconazole | 200 twice daily | 150 once daily | Ritonavir 100 once daily | 18 | 1.17 (1.04,1.33) | 1.48 (1.36,1.62) | 1.67 (1.48,1.88) |
| Ledipasvir/ Sofosbuvir | 90/400 once daily | 150 once daily‡ | Cobicistat 150 once daily‡ | 30 | 0.98 (0.90,1.07) | 1.11 (1.02,1.20) | 1.46 (1.28,1.66) |
| Omeprazole | 40 once daily given 2 hours before elvitegravir | 50 once daily | Ritonavir 100 once daily | 9 | 0.93 (0.83,1.04) | 0.99 (0.91,1.07) | 0.94 (0.85,1.04) |
| 20 once daily given 2 hours before elvitegravir | 150 once daily | Cobicistat 150 once daily | 11 | 1.16 (1.04,1.30) | 1.10 (1.02,1.19) | 1.13 (0.96,1.34) |
|
| 20 once daily given 12 hours after elvitegravir | 11 | 1.03 (0.92,1.15) | 1.05 (0.93,1.18) | 1.10 (0.92,1.32) |
|||
| Rifabutin | 150 once every other day | 150 once daily | Cobicistat 150 once daily | 12 | 0.91 (0.84,0.99) | 0.79 (0.74,0.85) | 0.33 (0.27,0.40) |
| Rosuvastatin | 10 single dose | 150 once daily | Cobicistat 150 once daily | 10 | 0.94 (0.83,1.07) | 1.02 (0.91,1.14) | 0.98 (0.83,1.16) |
| Sertraline | 50 single dose | 150 once daily‡ | Cobicistat 150 once daily‡ | 19 | 0.88 (0.82,0.93) | 0.94 (0.89,0.98) | 0.99 (0.93,1.05) |
| Sofosbuvir/ Velpatasvir | 400/100 once daily | 150 once daily‡ | Cobicistat 150 once daily‡ | 24 | 0.87 (0.80,0.94) | 0.94 (0.88,1.00) | 1.08 (0.97,1.20) |
| Sofosbuvir/ Velpatasvir/ Voxilaprevir | 400/100/100 + 100 Voxilaprevir§ once daily | 150 once daily‡ | Cobicistat 150 once daily‡ | 29 | 0.79 (0.75,0.85) | 0.94 (0.88,1.00) | 1.32 (1.17,1.49) |
| Coadministered Drug | Dose of Coadministered Drug (mg) | Emtricitabine Dose (mg) | N | Mean Ratio of Emtricitabine Pharmacokinetic Parameters (90% CI); No effect = 1.00 |
||
|---|---|---|---|---|---|---|
| Cmax | AUC | Cmin | ||||
|
||||||
| Famciclovir | 500 single dose | 200 single dose | 12 | 0.90 (0.80,1.01) | 0.93 (0.87,0.99) | NC |
| Coadministered Drug | Dose of Coadministered Drug (mg) | TAF Dose (mg) | N | Mean Ratio of TAF Pharmacokinetic Parameters (90% CI); No effect = 1.00 |
||
|---|---|---|---|---|---|---|
| Cmax | AUC | Cmin | ||||
| NC = Not Calculated | ||||||
| Cobicistat | 150 once daily | 8 once daily | 12 | 2.83 (2.20,3.65) | 2.65 (2.29,3.07) | NC |
| Ledipasvir/ Sofosbuvir | 90/400 once daily | 10 once daily† | 30 | 0.90 (0.73,1.11) | 0.86 (0.78,0.95) | NC |
| Sertraline | 50 single dose | 10 once daily† | 19 | 1.00 (0.86,1.16) | 0.96 (0.89,1.03) | NC |
| Sofosbuvir/ Velpatasvir | 400/100 once daily | 10 once daily† | 24 | 0.80 (0.68,0.94) | 0.87 (0.81,0.94) | NC |
| Sofosbuvir/ Velpatasvir/ Voxilaprevir | 400/100/100 + 100 Voxilaprevir‡ once daily | 10 once daily† | 29 | 0.79 (0.68,0.92) | 0.93 (0.85,1.01) | NC |
| Coadministered Drug | Dose of Coadministered Drug (mg) | Elvitegravir Dose (mg) | CYP3A Inhibitor Cobicistat Dose (mg) | FTC Dose (mg) | TAF Dose (mg) | N | Mean Ratio of Coadministered Drug Pharmacokinetic Parameters (90% CI); No effect = 1.00 |
||
|---|---|---|---|---|---|---|---|---|---|
| Cmax | AUC | Cmin | |||||||
| FTC = emtricitabine; TAF = tenofovir alafenamide | |||||||||
| N/A = Not Applicable; NC = Not Calculated | |||||||||
|
|||||||||
| Atorvastatin | 10 single dose | 150 once daily† | 150 once daily† | 200 once daily† | 10 once daily† | 16 | 2.32 (1.91,2.82) | 2.60 (2.31,2.93) | NC |
| Buprenorphine | 16 – 24 once daily | 150 once daily | 150 once daily | N/A | N/A | 17 | 1.12 (0.98,1.27) | 1.35 (1.18,1.55) | 1.66 (1.43,1.93) |
| Norbuprenorphine | 1.24 (1.03,1.49) | 1.42 (1.22,1.67) | 1.57 (1.31,1.88) |
||||||
| Carbamazepine | 200 twice daily | 150 once daily | 150 once daily | N/A | N/A | 12 | 1.40 (1.32,1.49) | 1.43 (1.36,1.52) | 1.51 (1.41,1.62) |
| Carbamazepine-10,11-epoxide | 0.73 (0.70,0.78) | 0.65 (0.63,0.66) | 0.59 (0.57,0.61) |
||||||
| Desipramine | 50 single dose | N/A | 150 once daily | N/A | N/A | 8 | 1.24 (1.08,1.44) | 1.65 (1.36,2.02) | NC |
| Digoxin | 0.5 single dose | N/A | 150 once daily | N/A | N/A | 22 | 1.41 (1.29,1.55) | 1.08 (1.00,1.17) | NC |
| Famciclovir | 500 single dose | N/A | N/A | 200 single dose | N/A | 12 | 0.93 (0.78,1.11) | 0.91 (0.84,0.99) | N/A |
| Ledipasvir | 90 once daily | 150 once daily† | 150 once daily† | 200 once daily† | 10 once daily† | 30 | 1.65 (1.53,1.78) | 1.79 (1.64,1.96) | 1.93 (1.74,2.15) |
| Sofosbuvir | 400 once daily | 1.28 (1.13,1.47) | 1.47 (1.35,1.59) | N/A | |||||
| GS-331007‡ | 1.29 (1.24,1.35) | 1.48 (1.44,1.53) | 1.66 (1.60,1.73) |
||||||
| Naloxone | 4–6 once daily | 150 once daily | 150 once daily | N/A | N/A | 17 | 0.72 (0.61,0.85) | 0.72 (0.59,0.87) | N/A |
| Norgestimate/ ethinyl estradiol§ | 0.180/0.215/ 0.250 norgestimate once daily | 150 once daily§ | 150 once daily§ | 200 once daily§ | N/A | 13 | 2.08 (2.00,2.17) | 2.26 (2.15,2.37) | 2.67 (2.43,2.92) |
| 0.025 ethinyl estradiol once daily | 0.94 (0.86,1.04) | 0.75 (0.69,0.81) | 0.56 (0.52,0.61) |
||||||
| Norgestromin | 0.180/0.215/ 0.250 norgestimate once daily / 0.025 ethinyl estradiol once daily | N/A | N/A | 200 once daily¶ | 25 once daily¶ | 15 | 1.17 (1.07,1.26) | 1.12 (1.07,1.17) | 1.16 (1.08,1.24) |
| Norgestrel | 1.10 (1.02,1.18) | 1.09 (1.01,1.18) | 1.11 (1.03,1.20) |
||||||
| Ethinyl estradiol | 1.22 (1.15,1.29) | 1.11 (1.07,1.16) | 1.02 (0.92,1.12) |
||||||
| R-Methadone | 80–120 daily | 150 once daily | 150 once daily | N/A | N/A | 11 | 1.01 (0.91,1.13) | 1.07 (0.96,1.19) | 1.10 (0.95,1.28) |
| S-Methadone | 0.96 (0.87,1.06) | 1.00 (0.89,1.12) | 1.02 (0.89,1.17) |
||||||
| Sertraline | 50 single dose | 150 once daily† | 150 once daily† | 200 once daily† | 10 once daily† | 19 | 1.14 (0.94,1.38) | 0.93 (0.77,1.13) | N/A |
| Rifabutin | 150 once every other day | 150 once daily | 150 once daily | N/A | N/A | 12 | 1.09 (0.98,1.20)# | 0.92 (0.83,1.03)# | 0.94 (0.85,1.04)# |
| 25-O-desacetyl-rifabutin | 12 | 4.84 (4.09,5.74)# | 6.25 (5.08,7.69)# | 4.94 (4.04,6.04)# |
|||||
| Rosuvastatin | 10 single dose | 150 once daily | 150 once daily | N/A | N/A | 10 | 1.89 (1.48,2.42) | 1.38 (1.14,1.67) | NC |
| Sofosbuvir | 400 once daily | 150 once daily† | 150 once daily† | 200 once daily† | 10 once daily† | 24 | 1.23 (1.07,1.42) | 1.37 (1.24,1.52) | N/A |
| GS-331007‡ | 1.29 (1.25,1.33) | 1.48 (1.43,1.53) | 1.58 (1.52,1.65) |
||||||
| Velpatasvir | 100 once daily | 1.30 (1.17,1.45) | 1.50 (1.35,1.66) | 1.60 (1.44,1.78) | |||||
| Sofosbuvir | 400 once daily | 150 once daily† | 150 once daily† | 200 once daily† | 10 once daily† | 29 | 1.27 (1.09,1.48) | 1.22 (1.12,1.32) | NC |
| GS-331007‡ | 1.28 (1.25,1.32) | 1.43 (1.39,1.47) | NC | ||||||
| Velpatasvir | 100 once daily | 0.96 (0.89,1.04) | 1.16 (1.06,1.27) | 1.46 (1.30,1.64) |
|||||
| Voxilaprevir | 100 + 100Þ once daily | 1.92 (1.63,2.26) | 2.71 (2.30,3.19) | 4.50 (3.68,5.50) |
|||||
12.4 Microbiology
Mechanism of Action
Elvitegravir: Elvitegravir inhibits the strand transfer activity of HIV-1 integrase (integrase strand transfer inhibitor; INSTI), an HIV-1 encoded enzyme that is required for viral replication. Inhibition of integrase prevents the integration of HIV-1 DNA into host genomic DNA, blocking the formation of the HIV-1 provirus and propagation of the viral infection. Elvitegravir does not inhibit human topoisomerases I or II.
Cobicistat: Cobicistat is a selective, mechanism-based inhibitor of cytochromes P450 of the CYP3A subfamily. Inhibition of CYP3A-mediated metabolism by cobicistat enhances the systemic exposure of CYP3A substrates, such as elvitegravir, where bioavailability is limited and half-life is shortened by CYP3A-dependent metabolism.
Emtricitabine: Emtricitabine, a synthetic nucleoside analog of cytidine, is phosphorylated by cellular enzymes to form emtricitabine 5'-triphosphate. Emtricitabine 5'-triphosphate inhibits the activity of the HIV-1 reverse transcriptase by competing with the natural substrate deoxycytidine 5'-triphosphate and by being incorporated into nascent viral DNA which results in chain termination. Emtricitabine 5'-triphosphate is a weak inhibitor of mammalian DNA polymerases α, β, Ɛ, and mitochondrial DNA polymerase γ.
Tenofovir Alafenamide (TAF): TAF is a phosphonamidate prodrug of tenofovir (2'-deoxyadenosine monophosphate analog). Plasma exposure to TAF allows for permeation into cells and then TAF is intracellularly converted to tenofovir through hydrolysis by cathepsin A. Tenofovir is subsequently phosphorylated by cellular kinases to the active metabolite tenofovir diphosphate. Tenofovir diphosphate inhibits HIV-1 replication through incorporation into viral DNA by the HIV reverse transcriptase, which results in DNA chain-termination.
Tenofovir has activity that is specific to human immunodeficiency virus and hepatitis B virus. Cell culture studies have shown that both emtricitabine and tenofovir can be fully phosphorylated when combined in cells. Tenofovir diphosphate is a weak inhibitor of mammalian DNA polymerases that include mitochondrial DNA polymerase γ and there is no evidence of mitochondrial toxicity in cell culture based on several assays including mitochondrial DNA analyses.
Antiviral Activity in Cell Culture
Elvitegravir, Cobicistat, Emtricitabine, and Tenofovir Alafenamide (TAF): The combination of elvitegravir, emtricitabine, and TAF was not antagonistic in cell culture combination antiviral activity assays and was not affected by the addition of cobicistat. In addition, elvitegravir, cobicistat, emtricitabine, and TAF were not antagonistic with a panel of representatives from the major classes of approved anti-HIV-1 agents (INSTIs, NNRTIs, NRTIs, and PIs).
Elvitegravir: The antiviral activity of elvitegravir against laboratory and clinical isolates of HIV-1 was assessed in T lymphoblastoid cell lines, monocyte/macrophage cells, and primary peripheral blood lymphocytes. The 50% effective concentrations (EC50) ranged from 0.02 to 1.7 nM. Elvitegravir displayed antiviral activity in cell culture against HIV-1 clades A, B, C, D, E, F, G, and O (EC50 values ranged from 0.1 to 1.3 nM) and activity against HIV-2 (EC50 value of 0.53 nM). Elvitegravir did not show inhibition of replication of HBV or HCV in cell culture.
Cobicistat: Cobicistat has no detectable antiviral activity in cell culture against HIV-1, HBV, or HCV and does not antagonize the antiviral activity of elvitegravir, emtricitabine, or tenofovir.
Emtricitabine: The antiviral activity of emtricitabine against laboratory and clinical isolates of HIV-1 was assessed in T lymphoblastoid cell lines, the MAGI-CCR5 cell line, and primary peripheral blood mononuclear cells. The EC50 values for emtricitabine were in the range of 0.0013–0.64 microM. Emtricitabine displayed antiviral activity in cell culture against HIV-1 clades A, B, C, D, E, F, and G (EC50 values ranged from 0.007–0.075 microM) and showed strain specific activity against HIV-2 (EC50 values ranged from 0.007–1.5 microM).
Tenofovir Alafenamide (TAF): The antiviral activity of TAF against laboratory and clinical isolates of HIV-1 subtype B was assessed in lymphoblastoid cell lines, PBMCs, primary monocyte/macrophage cells and CD4-T lymphocytes. The EC50 values for TAF ranged from 2.0 to 14.7 nM.
TAF displayed antiviral activity in cell culture against all HIV-1 groups (M, N, O), including sub-types A, B, C, D, E, F, and G (EC50 values ranged from 0.10 to 12.0 nM) and strain specific activity against HIV-2 (EC50 values ranged from 0.91 to 2.63 nM).
Resistance
In Cell Culture
Elvitegravir: HIV-1 isolates with reduced susceptibility to elvitegravir have been selected in cell culture. Reduced susceptibility to elvitegravir was associated with the primary integrase substitutions T66A/I, E92G/Q, S147G, and Q148R. Additional integrase substitutions observed in cell culture selection included D10E, S17N, H51Y, F121Y, S153F/Y, E157Q, D232N, R263K, and V281M.
Emtricitabine: HIV-1 isolates with reduced susceptibility to emtricitabine have been selected in cell culture. Reduced susceptibility to emtricitabine was associated with M184V or I substitutions in HIV-1 RT.
Tenofovir Alafenamide (TAF): HIV-1 isolates with reduced susceptibility to TAF have been selected in cell culture. HIV-1 isolates selected by TAF expressed a K65R substitution in HIV-1 RT, sometimes in the presence of S68N or L429I substitutions; in addition, a K70E substitution in HIV-1 RT was observed.
In Clinical Trials
In Treatment-Naïve Subjects:
In a pooled analysis of antiretroviral-naïve subjects receiving GENVOYA in Studies 104 and 111, genotyping was performed on plasma HIV-1 isolates from all subjects with HIV-1 RNA greater than 400 copies per mL at confirmed virologic failure, at Week 144, or at time of early study drug discontinuation. As of Week 144, the development of genotypic resistance to elvitegravir, emtricitabine, or TAF was observed in 12 of 22 subjects with evaluable resistance data from paired baseline and GENVOYA treatment-failure isolates (12 of 866 subjects [1.4%]) compared with 13 of 20 treatment-failure isolates from subjects with evaluable resistance data in the STRIBILD treatment group (13 of 867 subjects [1.5%]). Of the 12 subjects with resistance development in the GENVOYA group, the resistance-associated substitutions that emerged were M184V/I (N=11) and K65R/N (N=2) in reverse transcriptase and T66T/A/I/V (N=2), E92Q (N=4), E138K (N=1), Q148Q/R (N=1) and N155H (N=2) in integrase. Of the 13 subjects with resistance development in the STRIBILD group, the resistance-associated substitutions that emerged were M184V/I (N=9), K65R/N (N=4), and L210W (N=1) in reverse transcriptase and E92Q/V (N=4), E138K (N=3), Q148R (N=2), and N155H/S (N=3) in integrase. In both treatment groups, most subjects who developed substitutions associated with resistance to elvitegravir also developed emtricitabine resistance-associated substitutions. These genotypic resistance results were confirmed by phenotypic analyses.
In Virologically Suppressed Subjects:
Three virologic failure subjects were identified with emergent genotypic and phenotypic resistance to GENVOYA (all three with M184I or V and one with K219Q in reverse transcriptase; two with E92Q or G in integrase) out of 8 virologic failure subjects with resistance data in a clinical study of virologically-suppressed subjects who switched from a regimen containing emtricitabine/TDF and a third agent to GENVOYA (Study 109, N=959).
Cross-Resistance
No cross-resistance has been demonstrated for elvitegravir-resistant HIV-1 isolates and emtricitabine or tenofovir, or for emtricitabine- or tenofovir-resistant isolates and elvitegravir.
Elvitegravir: Cross-resistance has been observed among INSTIs. Elvitegravir-resistant viruses showed varying degrees of cross-resistance in cell culture to raltegravir depending on the type and number of amino acid substitutions in HIV-1 integrase. Of the primary elvitegravir resistance-associated substitutions tested (T66A/I/K, E92G/Q, T97A, S147G, Q148H/K/R, and N155H), all but three (T66I, E92G, and S147G) conferred greater than 1.5-fold reduced susceptibility to raltegravir (above the biological cutoff for raltegravir) when introduced individually into a wild-type virus by site-directed mutagenesis. Of the primary raltegravir resistance-associated substitutions (Y143C/H/R, Q148H/K/R, and N155H), all but Y143C/H conferred greater than 2.5-fold reductions in susceptibility to elvitegravir (above the biological cutoff for elvitegravir). Some viruses expressing elvitegravir or raltegravir resistance amino acid substitutions maintain susceptibility to dolutegravir.
Emtricitabine: Cross-resistance has been observed among NRTIs. Emtricitabine-resistant isolates harboring an M184V/I substitution in HIV-1 RT were cross-resistant to lamivudine. HIV-1 isolates containing the K65R RT substitution, selected in vivo by abacavir, didanosine, and tenofovir, demonstrated reduced susceptibility to inhibition by emtricitabine.
Tenofovir Alafenamide (TAF): Tenofovir resistance substitutions, K65R and K70E, result in reduced susceptibility to abacavir, didanosine, emtricitabine, lamivudine, and tenofovir.
HIV-1 with multiple TAMs (M41L, D67N, K70R, L210W, T215F/Y, K219Q/E/N/R), or multinucleoside resistant HIV-1 with a T69S double insertion mutation or with a Q151M mutation complex including K65R, showed reduced susceptibility to TAF in cell culture.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Elvitegravir
Long-term carcinogenicity studies of elvitegravir were carried out in mice (104 weeks) and in rats for up to 88 weeks (males) and 90 weeks (females). No drug-related increases in tumor incidence were found in mice at doses up to 2000 mg per kg per day alone or in combination with 25 mg per kg per day RTV at exposures 3- and 14 times, respectively, the human systemic exposure at the recommended daily dose of 150 mg. No drug-related increases in tumor incidence were found in rats at doses up to 2000 mg per kg per day at exposures 12- to 27 times, respectively in male and female, the human systemic exposure.
Elvitegravir was not genotoxic in the reverse mutation bacterial test (Ames test) and the rat micronucleus assay. In an in vitro chromosomal aberration test, elvitegravir was negative with metabolic activation; however, an equivocal response was observed without activation.
Elvitegravir did not affect fertility in male and female rats at approximately 16- and 30 times higher exposures (AUC), respectively, than in humans at the recommended 150 mg daily dose.
Fertility was normal in the offspring of rats exposed daily from before birth (in utero) through sexual maturity at daily exposures (AUC) of approximately 18 times higher than human exposures at the recommended 150 mg daily dose.
Cobicistat
In a long-term carcinogenicity study in mice, no drug-related increases in tumor incidence were observed at doses up to 50 and 100 mg/kg/day (males and females, respectively). Cobicistat exposures at these doses were approximately 7 (male) and 16 (females) times, respectively, the human systemic exposure at the therapeutic daily dose. In a long-term carcinogenicity study of cobicistat in rats, an increased incidence of follicular cell adenomas and/or carcinomas in the thyroid gland was observed at doses of 25 and 50 mg/kg/day in males, and at 30 mg/kg/day in females. The follicular cell findings are considered to be rat-specific, secondary to hepatic microsomal enzyme induction and thyroid hormone imbalance, and are not relevant for humans. At the highest doses tested in the rat carcinogenicity study, systemic exposures were approximately 2 times the human systemic exposure at the recommended daily dose.
Cobicistat was not genotoxic in the reverse mutation bacterial test (Ames test), mouse lymphoma or rat micronucleus assays.
Cobicistat did not affect fertility in male or female rats at daily exposures (AUC) approximately 4 times higher than human exposures at the recommended 150 mg daily dose.
Fertility was normal in the offspring of rats exposed daily from before birth (in utero) through sexual maturity at daily exposures (AUC) of approximately 1.2 times higher than human exposures at the recommended 150 mg daily dose.
Emtricitabine
In long-term carcinogenicity studies of emtricitabine, no drug-related increases in tumor incidence were found in mice at doses up to 750 mg per kg per day (23 times the human systemic exposure at the therapeutic dose of 200 mg per day) or in rats at doses up to 600 mg per kg per day (28 times the human systemic exposure at the recommended dose).
Emtricitabine was not genotoxic in the reverse mutation bacterial test (Ames test), mouse lymphoma or mouse micronucleus assays.
Emtricitabine did not affect fertility in male rats at approximately 140 times or in male and female mice at approximately 60 times higher exposures (AUC) than in humans given the recommended 200 mg daily dose. Fertility was normal in the offspring of mice exposed daily from before birth (in utero) through sexual maturity at daily exposures (AUC) of approximately 60 times higher than human exposures at the recommended 200 mg daily dose.
Tenofovir Alafenamide (TAF)
Since TAF is rapidly converted to tenofovir and a lower tenofovir exposure in rats and mice is observed after TAF administration compared to TDF administration, carcinogenicity studies were conducted only with TDF. Long-term oral carcinogenicity studies of TDF in mice and rats were carried out at exposures up to approximately 10 times (mice) and 4 times (rats) those observed in humans at the 300 mg therapeutic dose of TDF for HIV-1 infection. The tenofovir exposure in these studies was approximately 167 times (mice) and 55 times (rat) those observed in humans after administration of GENVOYA treatment. At the high dose in female mice, liver adenomas were increased at tenofovir exposures 10 times (300 mg TDF) and 167 times (10 mg TAF in GENVOYA) that in humans. In rats, the study was negative for carcinogenic findings.
TAF was not genotoxic in the reverse mutation bacterial test (Ames test), mouse lymphoma or rat micronucleus assays.
There were no effects on fertility, mating performance or early embryonic development when TAF was administered to male rats at a dose equivalent to 155 times the human dose based on body surface area comparisons for 28 days prior to mating and to female rats for 14 days prior to mating through Day 7 of gestation.
13.2 Animal Toxicology and/or Pharmacology
Minimal to slight infiltration of mononuclear cells in the posterior uvea was observed in dogs with similar severity after three and nine month administration of TAF; reversibility was seen after a three month recovery period. At the NOAEL for eye toxicity, the systemic exposure in dogs was 5 (TAF) and 15 (tenofovir) times the exposure seen in humans at the recommended daily GENVOYA dosage.
14 CLINICAL STUDIES
14.1 Description of Clinical Trials
The efficacy and safety of GENVOYA were evaluated in the studies summarized in Table 15.
| Trial | Population | Study Arms (N) | Timepoint (Week) |
|---|---|---|---|
|
|||
| Study 104*
Study 111* | Treatment-naïve adults | GENVOYA (866) STRIBILD (867) | 144 |
| Study 109† | Virologically-suppressed‡ adults | GENVOYA (959) ATRIPLA® or TRUVADA®+atazanavir+cobicistat or ritonavir or STRIBILD (477) | 96 |
| Study 112§ | Virologically-suppressed‡ adults with renal impairment¶ | GENVOYA (242) | 144 |
| Study 1825§ | Virologically-suppressed‡ adults with ESRD# receiving chronic hemodialysis | GENVOYA (55) | 48 |
| Study 106 (cohort 1)§ | Treatment-naïve adolescents between the ages of 12 to less than 18 years (at least 35 kg) | GENVOYA (50) | 48 |
| Study 106 (cohort 2)§ | Virologically-suppressed children between the ages of 6 to less than 12 years (at least 25 kg) | GENVOYA (23) | 24 |
14.2 Clinical Trial Results in HIV-1 Treatment-Naïve Subjects
In both Study 104 and Study 111, subjects were randomized in a 1:1 ratio to receive either GENVOYA (N=866) once daily or STRIBILD (elvitegravir 150 mg, cobicistat 150 mg, emtricitabine 200 mg, TDF 300 mg) (N=867) once daily. The mean age was 36 years (range 18–76), 85% were male, 57% were White, 25% were Black, and 10% were Asian. Nineteen percent of subjects identified as Hispanic/Latino. The mean baseline plasma HIV-1 RNA was 4.5 log10 copies per mL (range 1.3–7.0) and 23% of subjects had baseline viral loads greater than 100,000 copies per mL. The mean baseline CD4+ cell count was 427 cells per mm3 (range 0–1360) and 13% had CD4+ cell counts less than 200 cells per mm3.
Pooled treatment outcomes of Studies 104 and 111 through Week 144 are presented in Table 16.
| GENVOYA (N=866) | STRIBILD (N=867) |
|
|---|---|---|
|
||
| HIV-1 RNA < 50 copies/mL† | 84% | 80% |
| HIV-1 RNA ≥ 50 copies/mL‡ | 5% | 4% |
| No Virologic Data at Week 144 Window | 11% | 16% |
| Discontinued Study Drug Due to AE or Death§ | 2% | 3% |
| Discontinued Study Drug Due to Other Reasons and Last Available HIV-1 RNA < 50 copies/mL¶ | 9% | 11% |
| Missing Data During Window but on Study Drug | 1% | 1% |
Treatment outcomes were similar across subgroups by age, sex, race, baseline viral load, and baseline CD4+ cell count.
In Studies 104 and 111, the mean increase from baseline in CD4+ cell count at Week 144 was 326 cells per mm3 in GENVOYA-treated subjects and 305 cells per mm3 in STRIBILD-treated subjects.
14.3 Clinical Trial Results in HIV-1 Virologically-Suppressed Subjects Who Switched to GENVOYA
In Study 109, the efficacy and safety of switching from ATRIPLA, TRUVADA plus atazanavir (given with either cobicistat or ritonavir), or STRIBILD to GENVOYA once daily were evaluated in a randomized, open-label trial of virologically-suppressed (HIV-1 RNA less than 50 copies per mL) HIV-1 infected adults (N=1436). Subjects must have been suppressed (HIV-1 RNA less than 50 copies per mL) on their baseline regimen for at least 6 months and had no known resistance-associated substitutions to any of the components of GENVOYA prior to study entry. Subjects were randomized in a 2:1 ratio to either switch to GENVOYA at baseline (N=959), or stay on their baseline antiretroviral regimen (N=477). Subjects had a mean age of 41 years (range 21–77), 89% were male, 67% were White, and 19% were Black. The mean baseline CD4+ cell count was 697 cells per mm3 (range 79–1951).
Subjects were stratified by prior treatment regimen. At screening, 42% of subjects were receiving TRUVADA plus atazanavir (given with either cobicistat or ritonavir), 32% were receiving STRIBILD, and 26% were receiving ATRIPLA.
Treatment outcomes of Study 109 through 96 weeks are presented in Table 17.
| GENVOYA (N=959) | ATRIPLA or TRUVADA+atazanavir +cobicistat or ritonavir or STRIBILD (N=477) |
|
|---|---|---|
|
||
| HIV-1 RNA < 50 copies/mL | 93% | 89% |
| HIV-1 RNA ≥ 50 copies/mL† | 2% | 2% |
| No Virologic Data at Week 48 Window | 5% | 9% |
| Discontinued Study Drug Due to AE or Death‡ | 1% | 3% |
| Discontinued Study Drug Due to Other Reasons and Last Available HIV-1 RNA < 50 copies/mL§ | 3% | 6% |
| Missing Data During Window but on Study Drug | 1% | <1% |
Treatment outcomes were similar across subgroups receiving ATRIPLA, TRUVADA plus atazanavir (given with either cobicistat or ritonavir), or STRIBILD prior to randomization. In Study 109, the mean increase from baseline in CD4+ cell count at Week 96 was 60 cells per mm3 in GENVOYA-treated subjects and 42 cells per mm3 in subjects who stayed on their baseline regimen.
14.4 Clinical Trial Results in HIV-1 Infected Subjects with Renal Impairment
Study 112: Virologically-suppressed adults with renal impairment
In Study 112, the efficacy and safety of GENVOYA once daily were evaluated in an open-label clinical trial of 248 HIV-1 infected subjects with renal impairment (estimated creatinine clearance between 30 and 69 mL per minute by Cockcroft-Gault method). Of the 248 enrolled, 6 were treatment-naïve and 242 were virologically suppressed (HIV-1 RNA less than 50 copies per mL) for at least 6 months before switching to GENVOYA [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)].
The mean age was 58 years (range 24–82), with 63 subjects (26%) who were 65 years of age or older. Seventy-nine percent were male, 63% were White, 18% were Black, and 14% were Asian. Thirteen percent of subjects identified as Hispanic/Latino. The mean baseline CD4+ cell count was 664 cells per mm3 (range 126–1813). At Week 144, 81% (197/242 virologically suppressed subjects) maintained HIV-1 RNA less than 50 copies per mL after switching to GENVOYA. All six treatment-naïve subjects were virologically suppressed at Week 144. Five subjects among the entire study population had virologic failure at Week 144.
Study 1825: Virologically-suppressed adults with end stage renal disease (ESRD) receiving chronic hemodialysis
In Study 1825, the efficacy and safety of GENVOYA once daily were evaluated in an open-label clinical trial of 55 virologically-suppressed (HIV-1 RNA less than 50 copies per mL for at least 6 months before switching to GENVOYA) HIV-1 infected subjects with ESRD (estimated creatinine clearance of less than 15 mL per minute by Cockcroft-Gault method) receiving chronic hemodialysis for at least 6 months [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)].
Subjects had a mean age of 48 years (range 23–64), 76% were male, 82% were Black, 18% were White, and 15% identified as Hispanic/Latino. The mean baseline CD4+ cell count was 545 cell per mm3 (range 205–1473). At Week 48, 82% (45/55) maintained HIV-1 RNA less than 50 copies per mL after switching to GENVOYA. Two subjects had HIV-1 RNA ≥ 50 copies per mL by Week 48. Seven subjects discontinued the study drug due to AE or other reasons while suppressed. One subject did not have an HIV-1 RNA measurement at Week 48.
14.5 Clinical Trial Results in HIV-1 Infected Pediatric Subjects Between the Ages of 6 to Less than 18
In Study 106, an open-label, single arm trial the efficacy, safety, and pharmacokinetics of GENVOYA in HIV-1 infected pediatric subjects were evaluated in treatment-naïve adolescents between the ages of 12 to less than 18 years weighing at least 35 kg (N=50) and in virologically-suppressed children between the ages of 6 to less than 12 years weighing at least 25 kg (N=23).
Cohort 1: Treatment-naïve adolescents (12 to less than 18 years; at least 35 kg)
Subjects in cohort 1 treated with GENVOYA once daily had a mean age of 15 years (range 12-17); 44% were male, 12% were Asian, and 88% were Black. At baseline, mean plasma HIV-1 RNA was 4.6 log10 copies per mL (22% had baseline plasma HIV-1 RNA greater than 100,000 copies per mL), median CD4+ cell count was 456 cells per mm3 (range: 95 to 1110), and median CD4+ percentage was 23% (range: 7% to 45%).
In subjects in cohort 1 treated with GENVOYA, 92% (46/50) achieved HIV-1 RNA less than 50 copies per mL at Week 48. The mean increase from baseline in CD4+ cell count at Week 48 was 224 cells per mm3. Three of 50 subjects had virologic failure at Week 48; no emergent resistance to GENVOYA was detected through Week 48.
Cohort 2: Virologically-suppressed children (6 to less than 12 years; at least 25 kg)
Subjects in cohort 2 treated with GENVOYA once daily had a mean age of 10 years (range: 8–11), a mean baseline weight of 31.6 kg, 39% were male, 13% were Asian, and 78% were Black. At baseline, median CD4+ cell count was 969 cells/mm3 (range: 603 to 1421), and median CD4% was 39% (range: 30% to 51%).
After switching to GENVOYA, 100% (23/23) of subjects in cohort 2 remained suppressed (HIV-1 RNA < 50 copies/mL) at Week 24. From a mean (SD) baseline CD4+ cell count of 966 (201.7), the mean change from baseline in CD4+ cell count was -150 cells/mm3 and the mean (SD) change in CD4% was -1.5% (3.7%) at Week 24. All subjects maintained CD4+ cell counts above 400 cells/mm3 [see Adverse Reactions (6.1) and Pediatric Use (8.4)].
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Drug Interactions
GENVOYA may interact with many drugs; therefore, advise patients to report to their healthcare provider the use of any other prescription or non-prescription medication or herbal products including St. John's wort [see Contraindications (4) and Drug Interactions (7)].
Post-treatment Acute Exacerbation of Hepatitis B in Patients with HBV Co-Infection
Severe acute exacerbations of hepatitis B have been reported in patients who are coinfected with HBV and HIV-1 and have discontinued products containing emtricitabine and/or TDF, and may likewise occur with discontinuation of GENVOYA [see Warnings and Precautions (5.1)]. Advise the patient to not discontinue GENVOYA without first informing their healthcare provider.
Immune Reconstitution Syndrome
Advise patients to inform their healthcare provider immediately of any symptoms of infection, as in some patients with advanced HIV infection (AIDS), signs and symptoms of inflammation from previous infections may occur soon after anti-HIV treatment is started [see Warnings and Precautions (5.3)].
Renal Impairment
Advise patients to avoid taking GENVOYA with concurrent or recent use of nephrotoxic agents. Renal impairment including cases of acute renal failure has been reported in association with the use of tenofovir prodrugs [see Warnings and Precautions (5.4)].
Lactic Acidosis and Severe Hepatomegaly
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with use of drugs similar to GENVOYA. Advise patients that they should stop GENVOYA if they develop clinical symptoms suggestive of lactic acidosis or pronounced hepatotoxicity [see Warnings and Precautions (5.5)].
Missed Dosage
Inform patients that it is important to take GENVOYA on a regular dosing schedule with food and to avoid missing doses as it can result in development of resistance [see Dosage and Administration (2.2)].
Pregnancy
Advise patients that GENVOYA is not recommended during pregnancy and to alert their healthcare provider if they become pregnant while taking GENVOYA [see Dosage and Administration (2.5) and Use in Specific Populations (8.1)]. Inform patients that there is an antiretroviral pregnancy registry to monitor fetal outcomes of pregnant individuals exposed to GENVOYA [see Use in Specific Populations (8.1)].
Lactation
Instruct patients with HIV-1 infection not to breastfeed because HIV-1 can be passed to the baby in breast milk [see Use in Specific Populations (8.2)].
| This Patient Information has been approved by the U.S. Food and Drug Administration | Revised: 12/2018 |
| Patient Information | |
| GENVOYA® (jen-VOY-uh) (elvitegravir, cobicistat, emtricitabine, and tenofovir alafenamide) tablets |
|
|
Important: Ask your healthcare provider or pharmacist about medicines that should not be taken with GENVOYA. For more information, see the section "What should I tell my healthcare provider before taking GENVOYA?" |
|
|
What is the most important information I should know about GENVOYA? GENVOYA can cause serious side effects, including:
For more information about side effects, see "What are the possible side effects of GENVOYA?" |
|
|
What is GENVOYA? GENVOYA is a prescription medicine that is used without other antiviral medicines to treat Human Immunodeficiency Virus-1 (HIV-1) in adults and children who weigh at least 55 pounds (25 kg):
HIV-1 is the virus that causes AIDS (Acquired Immune Deficiency Syndrome). GENVOYA contains the prescription medicines elvitegravir, cobicistat, emtricitabine and tenofovir alafenamide. It is not known if GENVOYA is safe and effective in children who weigh less than 55 pounds (25 kg). |
|
| Do not take GENVOYA if you also take a medicine that contains: | |
|
|
|
|
|
What should I tell my healthcare provider before taking GENVOYA? Before taking GENVOYA, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Some medicines may interact with GENVOYA. Keep a list of your medicines and show it to your healthcare provider and pharmacist when you get a new medicine.
|
|
|
How should I take GENVOYA?
|
|
|
What are the possible side effects of GENVOYA? GENVOYA may cause serious side effects, including:
The most common side effect of GENVOYA is nausea. These are not all the possible side effects of GENVOYA. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|
|
How should I store GENVOYA?
Keep GENVOYA and all medicines out of reach of children. |
|
|
General information about the safe and effective use of GENVOYA. Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use GENVOYA for a condition for which it was not prescribed. Do not give GENVOYA to other people, even if they have the same symptoms you have. It may harm them. You can ask your healthcare provider or pharmacist for information about GENVOYA that is written for health professionals. For more information, call 1-800-445-3235 or go to www.GENVOYA.com. |
|
|
What are the ingredients in GENVOYA? Active ingredients: elvitegravir, cobicistat, emtricitabine, and tenofovir alafenamide Inactive ingredients: croscarmellose sodium, hydroxypropyl cellulose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, silicon dioxide, and sodium lauryl sulfate. The tablets are film-coated with a coating material containing FD&C Blue No. 2/indigo carmine aluminum lake, iron oxide yellow, polyethylene glycol, polyvinyl alcohol, talc, and titanium dioxide. Manufactured and distributed by: Gilead Sciences, Inc. Foster City, CA 94404 GENVOYA is a trademark of Gilead Sciences, Inc., or its related companies. © 2019 Gilead Sciences, Inc. All rights reserved. 207561-GS-013 |
|
| GENVOYA
elvitegravir, cobicistat, emtricitabine, and tenofovir alafenamide tablet |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - A-S Medication Solutions (830016429) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| A-S Medication Solutions | 830016429 | RELABEL(50090-2279) | |
