Label: FLAREX- fluorometholone acetate suspension/ drops
- NDC Code(s): 71776-100-03, 71776-100-05
- Packager: Eyevance Pharmaceuticals
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated October 10, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
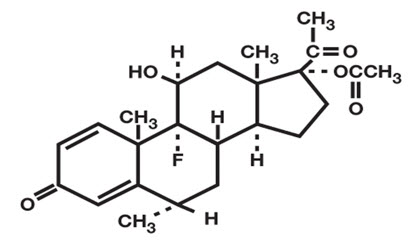
FLAREX ® (fluorometholone acetate ophthalmic suspension) 0.1% is a corticosteroid prepared as a sterile topical ophthalmic suspension. The active ingredient, fluorometholone acetate, is a white to creamy white powder with an empirical formula of C 24H 31FO 5 and a molecular weight of 418.5. Its chemical name is 9-fluoro-11β, 17-dihydroxy-6α-methylpregna-1, 4-diene-3, 20-dione 17-acetate. The chemical structure of Fluorometholone Acetate is presented below:
Each mL of FLAREX (fluorometholone acetate ophthalmic suspension) 0.1% contains: Active: fluorometholone acetate 1 mg (0.1%). Preservative: benzalkonium chloride 0.01%. Inactives: sodium chloride, monobasic sodium phosphate, edetate disodium, hydroxyethyl cellulose, tyloxapol, hydrochloric acid and/or sodium hydroxide (to adjust pH), and purified water. The pH of the suspension is approximately 7.3, with an osmolality of approximately 300 mOsm/kg.
-
CLINICAL PHARMACOLOGY
Corticosteroids suppress the inflammatory response to inciting agents of mechanical, chemical or immunological nature. No generally accepted explanation of this steroid property has been advanced. Corticosteroids cause a rise in intraocular pressure (IOP) in susceptible individuals. In a small study, FLAREX (fluorometholone acetate ophthalmic suspension) 0.1% demonstrated a significantly longer average time to produce a rise in IOP than did dexamethasone phosphate; however, the ultimate magnitude of the rise was equivalent for both drugs and in a small percentage of individuals a significant rise in IOP occurred within three days.
- INDICATIONS AND USAGE
-
CONTRAINDICATIONS
Contraindicated in acute superficial herpes simplex keratitis, vaccinia, varicella, and most other viral diseases of cornea and conjunctiva; mycobacterial infection of the eye; fungal diseases; acute purulent untreated infections, which like other diseases caused by microorganisms, may be masked or enhanced by the presence of the steroid; and in those persons who have known hypersensitivity to any component of this preparation.
-
WARNINGS
FOR TOPICAL OPHTHALMIC USE. NOT FOR INJECTION. Use in the treatment of herpes simplex infection requires great caution. Prolonged use may result in glaucoma, damage to the optic nerve, defect in visual acuity and visual field, cataract formation and/or may aid in the establishment of secondary ocular infections from pathogens due to suppression of host response. Acute purulent infections of the eye may be masked or exacerbated by presence of steroid medication. Topical ophthalmic corticosteroids may slow corneal wound healing. In those diseases causing thinning of the cornea or sclera, perforation has been known to occur with chronic use of topical steroids. If these products are used for 10 days or longer, intraocular pressure (IOP) should be routinely monitored even though it may be difficult in children and uncooperative patients.
-
PRECAUTIONS
General
Fungal infections of the cornea are particularly prone to develop coincidentally with long-term local steroid application. Fungus invasion must be considered in any persistent corneal ulceration where a steroid has been used or is in use.
Prescription Guidelines
The initial prescription and renewal of the medication order should be made by a physician only after examination of the patient with the aid of magnification, such as slit lamp biomicroscopy, and, where appropriate, fluorescein staining. If signs and symptoms fail to improve after 2 days, the patient should be re-evaluated.
Not more than one bottle should be prescribed initially, and the prescription should not be refilled without further evaluation.
Information for Patients
Do not touch dropper tip to any surface, as this may contaminate the suspension. The preservative in FLAREX ® (fluorometholone acetate ophthalmic suspension) 0.1%, benzalkonium chloride, may be absorbed by soft contact lenses. Contact lenses should be removed during instillation of FLAREX (fluorometholone acetate ophthalmic suspension) 0.1% but may be reinserted 15 minutes after instillation.
Patients should be advised that their vision may be temporarily blurred following dosing with FLAREX ® (fluorometholone acetate ophthalmic suspension) 0.1%. Care should be exercised in operating machinery or driving a motor vehicle.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No studies have been conducted in animals or in humans to evaluate the possibility of these effects with fluorometholone.
Pregnancy
Fluorometholone has been shown to be embryocidal and teratogenic in rabbits when administered at low multiples of the human ocular dose. Fluorometholone was applied ocularly to rabbits daily on days 6-18 of gestation, and dose-related fetal loss and fetal abnormalities including cleft palate, deformed rib cage, anomalous limbs and neural abnormalities such as encephalocele, craniorachischisis, and spina bifida were observed. There are no adequate and well controlled studies of fluorometholone in pregnant women, and it is not known whether fluorometholone can cause fetal harm when administered to a pregnant woman. Fluorometholone should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
Systemically administered corticosteroids appear in human milk and could suppress growth, interfere with endogenous corticosteroid production, or cause other untoward effects. It is not known whether topical administration of corticosteroids could result in sufficient systemic absorption to produce detectable quantities in human milk. Because many drugs are excreted in human milk, caution should be exercised when FLAREX (fluorometholone acetate ophthalmic suspension) 0.1%, is administered to a nursing woman.
-
ADVERSE REACTIONS
Glaucoma with optic nerve damage, visual acuity and field defects, cataract formation, secondary ocular infection following suppression of host response, and perforation of the globe may occur.
Postmarketing Experience
The following reaction has been identified during post-marketing use of FLAREX ® (fluorometholone acetate ophthalmic suspension) 0.1% in clinical practice. Because reactions are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. The reaction, which has been chosen for inclusion due to either its seriousness, frequency of reporting, possible causal connection to FLAREX, or a combination of these factors, includes: dysgeusia.
The following rare adverse reactions have been reported: Cushing's syndrome and adrenal suppression may occur after very frequent use of topical ophthalmic corticosteroids, particularly in very young children.
-
DOSAGE AND ADMINISTRATION
Shake Well Before Using. One to two drops instilled into the conjunctival sac(s) four times daily. During the initial 24 to 48 hours the dosage may be safely increased to two drops every two hours. If no improvement after two weeks, consult physician. Care should be taken not to discontinue therapy prematurely.
Not more than one bottle should be prescribed initially, and the prescription should not be refilled without further evaluation [see PRECAUTIONS] .
- HOW SUPPLIED
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 2.5 mL Bottle Carton
- PRINCIPAL DISPLAY PANEL - 5 mL Bottle Carton
-
INGREDIENTS AND APPEARANCE
FLAREX
fluorometholone acetate suspension/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:71776-100 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FLUOROMETHOLONE ACETATE (UNII: 9I50C3I3OK) (FLUOROMETHOLONE - UNII:SV0CSG527L) FLUOROMETHOLONE ACETATE 1 mg in 1 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM PHOSPHATE, MONOBASIC, UNSPECIFIED FORM (UNII: 3980JIH2SW) EDETATE DISODIUM (UNII: 7FLD91C86K) HYDROXYETHYL CELLULOSE (2000 MPA.S AT 1%) (UNII: S38J6RZN16) TYLOXAPOL (UNII: Y27PUL9H56) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71776-100-05 1 in 1 CARTON 03/05/2019 1 5 mL in 1 BOTTLE, PLASTIC; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 2 NDC:71776-100-03 1 in 1 CARTON 01/01/2022 2 2.5 mL in 1 BOTTLE, PLASTIC; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019079 03/05/2019 Labeler - Eyevance Pharmaceuticals (080876046) Establishment Name Address ID/FEI Business Operations Alcon Research LLC 007672236 manufacture(71776-100) Establishment Name Address ID/FEI Business Operations Sicor Societa' Italiana Corticosteroidi s.r.l 429369713 api manufacture(71776-100)





 Sample - Not for Sale
Sample - Not for Sale

 eyevance
eyevance
