VISINE ALLERGY EYE RELIEF MULTI-ACTION- naphazoline hydrochloride and pheniramine maleate solution/ drops
Johnson & Johnson Consumer Inc.
----------
Visine® Allergy Eye Relief Multi-Action
Warnings
For external use only
Ask a doctor before use if you have
- heart disease
- high blood pressure
- narrow angle glaucoma
- trouble urinating
When using this product
- pupils may become enlarged temporarily causing light sensitivity
- do not touch tip of container to any surface to avoid contamination
- replace cap after each use
- remove contact lenses before using
- do not use if this solution changes color or becomes cloudy
- overuse may cause more eye redness
- some users may experience a brief tingling sensation
Directions
- adults and children 6 years of age and over: put 1 or 2 drops in the affected eye(s) up to 4 times a day
- children under 6 years of age: consult a doctor
Inactive ingredients
benzalkonium chloride, boric acid, edetate disodium, purified water, sodium borate, sodium hydroxide and/or hydrochloric acid
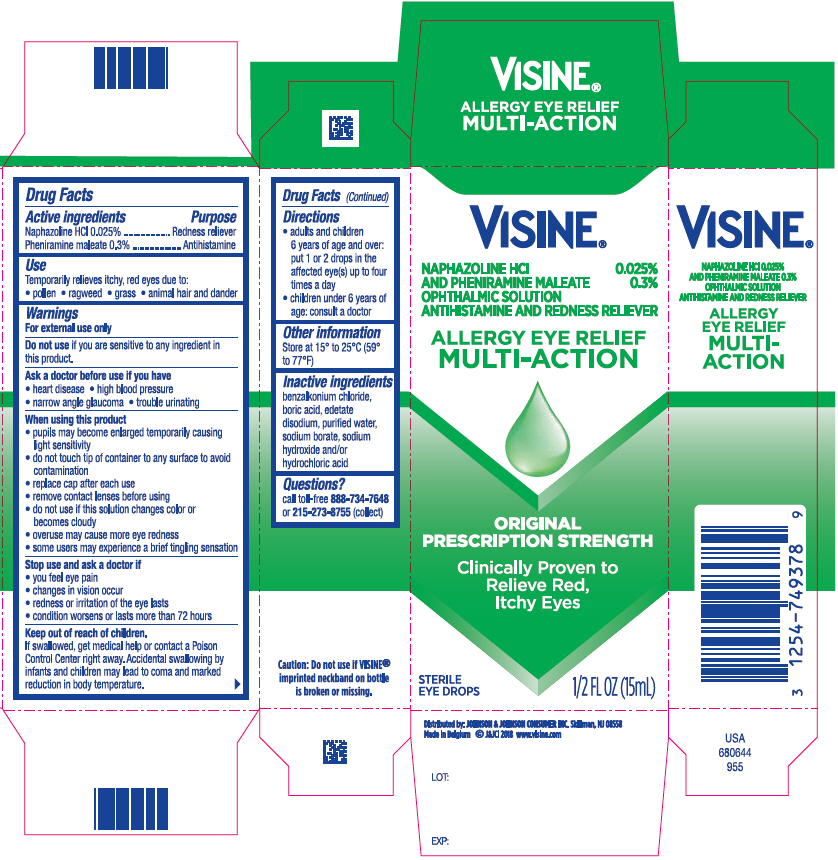
PRINCIPAL DISPLAY PANEL - 15 mL Bottle Carton
VISINE®
NAPHAZOLINE HCl 0.025%
AND PHENIRAMINE MALEATE 0.3%
OPHTHALMIC SOLUTION
ANTIHISTAMINE AND REDNESS RELIEVER
ALLERGY EYE RELIEF
MULTI-ACTION
ORIGINAL
PRESCRIPTION STRENGTH
Clinically Proven to
Relieve Red,
Itchy Eyes
STERILE
EYE DROPS
1/2 FL OZ (15mL)
| VISINE ALLERGY EYE RELIEF MULTI-ACTION
naphazoline hydrochloride and pheniramine maleate solution/ drops |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Johnson & Johnson Consumer Inc. (118772437) |
Revised: 1/2023
Document Id: ee86ce3e-844d-7d69-e053-2a95a90a748b
Set id: 125fbc97-5bc8-436e-9ee9-e5a44384e260
Version: 5
Effective Time: 20230104
Johnson & Johnson Consumer Inc.