Label: FLUDEOXYGLUCOSE F-18- fludeoxyglucose f-18 injection injection
-
Contains inactivated NDC Code(s)
NDC Code(s): 24468-001-10 - Packager: University of North Dakota
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 10, 2015
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Fludeoxyglucose F18 Injection, USP is a positron emitting radiopharmaceutical containing no-carrier added radioactive 2-deoxy-2-[ 18F]fluoro-D-glucose that is used for diagnostic purposes in conjunction with Positron Emission Tomography (PET). It is administered by intravenous injection.
The active ingredient 2-deoxy-2-[ 18F]fluoro-D-glucose, abbreviated [ 18F]FDG, has a molecular formula of C 8H 1118F0 8 with a molecular weight of 181.26 daltons, and has the following chemical structure:
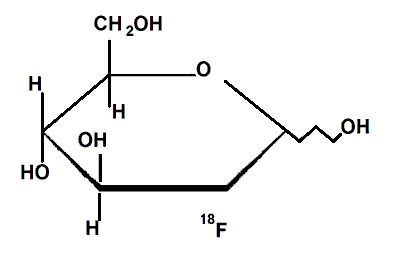
Fludeoxyglucose F18 Injection, USP is provided as a ready to use sterile, pyrogen free, clear, colorless solution. Each milliliter contain between 150 to 1850 MBq (4 — 500 mCi) of 2-deoxy-2-[ 18F]fluoro-D-glucose at the end of synthesis (EOS), and 9 mg of sodium chloride in citrate buffer. The pH of the solution is between 4.5 to 7.5. The solution is packaged in a multiple-dose glass vial and does not contain any preservative.
-
PHYSICAL CHARACTERISTICS
Fluorine F 18 decays by positron ( +) emission and has a half life of 110 minutes. The principal photons useful for diagnostic imaging are the 511 keV gamma photons, resulting from the interaction of the emitted positron with an electron (Table 1)
Table 1. Principle Emission Data for Fluorine F-18 Radiation/Emission % Per Disintigration Mean Energy Positron (+) 96.73 249.8 keV Gamma (+/-)* 193.46 511.0 keV *Produced by positron annihilation
From: Kocher, D.C. "Radioactive Decay Tables" DOE/TIC-11026, 89 (1981).
-
EXTERNAL RADIATION
The specific gamma ray constant for Fluorine F 18 is 6.0 R/hr/mCi (0.3 Gy/hr/kBq) at 1cm. The half-value layer (HVL) for the 511 keV photons is 4.1 mm lead (Pb). A range of values for the attenuation of radiation results from the interposition of various thicknesses of Pb.
The range of attenuation coefficients for this radionuclide is shown in Table 2. For example the interposition of an 8.3 mm thickness of Pb, with a coefficient of attenuation of 0.25, will decrease the external radiation by 75%.
Table 2. Radiation Attenuation of 511 keV Photons by Lead (Pb) Shielding Shield Thickness (Pb) mm Coefficient of Attenuation 0 0.00 4.1 0.50 8.3 0.25 13.2 0.10 26.4 0.01 52.8 0.001 For use in correcting for physical decay of this radionuclide, the fractions remaining at selected intervals after calibration are shown in Table 3.
-
CLINICAL PHARMACOLOGY
General
[ 18F]FDG, a radiolabeled analog of glucose, rapidly distributes, after intravenous injection, to all organs of the body. After background clearance, peak imaging is at 30 - 40 minutes after injection.
Table 3. Physical Decay Chart for Fluorine F-18 Minutes Fraction Remaining 0* 1.00 15 0.909 30 0.826 60 0.683 110 0.500 220 0.250 440 0.060 *Calibration Time
Pharmacokinetics
In 4 normal male volunteers, after an intravenous dose given over 30 seconds, the arterial blood level profile for [ 18F]FDG can be described by a triexponential decay curve. The half-lives for the different distribution and elimination phases are 0.2-0.3 min, 10-13 min (mean ± s.d.; 11.6 ± 1.1 min), and 80-95 min (88 ± 4 min).
Within 33 minutes, a mean of 3.9% of the injected dose can be measured in the urine. Bladder activity two hours after injection indicates that a mean of 20.6% of the injected dose is present. See the Metabolism Section for additional clearance times.
Metabolism
[ 18F]FDG is taken up by cells and phosphorylated to [ 18F]FDG-6-phosphate at a rate proportional to the rate of glucose utilization within a given tissue. [ 18F]FDG-6-phosphate presumably is metabolized to 2- deoxy-2-[ 18F]fluoro-6-phospho-D-mannose ([ 18F]FDM-6-phosphate).
[ 18F]FDG may contain 2-deoxy-2-chloro-D-glucose (C1DG) as an impurity. Distribution and metabolism of C1DG are presumably similar to that of [ 18F]FDG, and would be expected to result in intracellular formation of 2-deoxy-2-chloro-6-phospho-D-g14icose (C1DG-6-phosphate) and 2-deoxy-2-chloro-6- phospho-D-mannose (CIDM-6-phosphate). The phosphorylated deoxyglucose compounds are dephosphorylated, and the resulting compounds, (FDG, FDM, C1DG and C1DM) presumably leave cells by passive diffusion.
FDG and related compounds are cleared from non-cardiac tissues within 3 to 24 hours after administration; clearance from the heart may require more than 96 hours. [ 18F]FDG that is not involved in glucose metabolism is excreted unchanged in the urine.
Pharmacodynamics
[ 18F]FDG is a glucose analogue which concentrates in cells that rely upon glucose as a primary energy source. Once in the cell it is phosphorylated and can not exit until dephosphorylation has occurred. Regions of “increased [ 18F]FDG uptake" correlate with increased glucose metabolism. Regions of decreased/absent uptake reflect the absence of glucose metabolism. Background activity reflects uptake by normal cells. [ 18F]FDG uptake in inflammatory cells is inconsistent and may be increased, normal or decreased. Whether or not [ 18F]FDG, CIDG, or their metabolites can inhibit glucose metabolism is not known.
-
CLINCAL TRIALS
In a prospective, open label trial, [ 18F]FDG was evaluated in 86 patients with epilepsy. Each patient received a dose of [ 18F]FDG in the range of 185-370 MBq (5-10) mCi. Demographic characteristics of race and gender are not available. The mean age was 16.4 years (range: 4 months - 58 years; of these, 42 patients were < 12 years and 16 patients were <2 years old). Patients had a known diagnosis of complex partial epilepsy and were under evaluation as surgical candidates for treatment of their seizure disorder. Seizure foci had been previously identified on ictal EEGs and sphenoidal EEGs. In 16% (14/87) of patients, the pre-[ 18F]FDG findings were confirmed by [ 18F]FDG; 34% (30/87) of patients, [ 18F]FDG scans provided new findings. In 32% (27/87), [ 18F]FDG scans were not definitive. The influence of these findings on surgical outcome; medical management or behavior is not known.
In several other studies comparing [ 18F]FDG scan results to subsphenoidal EEG, MRI and/or surgical findings, the degree of hypometabolism corresponded to areas of confirmed epileptogenic foci.
-
INDICATIONS AND USAGE
Fludeoxyglucose F 18 Injection,USP is indicated in PET (positron emission tomography) for:
1. Identification of regions of abnormal glucose metabolism associated with foci of epileptic seizures.
2. Assessment of abnormal glucose metabolism to assist in the evaluation of malignancy in patients with known or suspected abnormalities found by other testing modalities, or in patients with an existing diagnosis of cancer.
3. Assessment of patients with coronary artery disease and left ventricular dysfunction, when used together with myocardial perfusion imaging, for the identification of left ventricular myocardium with residual glucose metabolism and reversible loss of systolic function.
Fludeoxyglucose F 18 Injection, USP is not indicated for distinguishing epileptogenic foci from brain tumors or other brain lesions which may cause seizures.
- WARNINGS
- CONTRAINDICATIONS
-
PRECAUTIONS
General
[ 18F]FDG uptake may be changed by fasting or by blood sugar changes associated with diabetic mellitus. Blood glucose levels should be stabilized in non-diabetic patients by fasting before [ 18F]FDG injection. Diabetic patients may need stabilization of blood glucose on the day preceding, and on the day of the [ 18F]FDG scan.
Patients should be monitored for arrhythmias and other manifestations of ischemia. [ 18F]FDG, CIDG and their metabolites theoretically could inhibit glucose metabolism. Their ability to potentiate the arrhythmogenic effects of ischemia has not been studied.
The contents of each vial are sterile and non-pyrogenic. To maintain sterility, aseptic technique must be used during all operations involved in the manipulation and administration of [ 18F]FDG.
[ 18F]FDG should be used within 12 hours of the end of synthesis (EOS).
As with any other radioactive material, appropriate shielding should be used to avoid unnecessary radiation exposure to the patient, occupational workers, and other persons.
Radiopharmaceuticals should be used only by physicians who are qualified by specific training in the safe use and handling of radionuclides.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies with [ 18F]FDG have not been performed to evaluate carcinogenic potential, mutagenic potential or effects on fertility.
Teratogenic Effects: Pregnancy Category C
Animal reproduction studies have not been conducted with [ 18F]FDG. It is not known whether [ 18F]FDG can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Therefore, [ 18F]FDG should not be administered to a pregnant woman unless the potential benefit justifies the potential risk to the fetus.
-
ADVERSE REACTIONS
The [ 18F]FDG safety data base was evaluated for 374 patients. Of these, 245 were male and 105 were female. For 24 patients, gender was not specified. The mean age was 47.8 years (range under 2 to over 65 years). Eighteen patients were between the age of 0 and 2 years; 42 patients were between the ages of 2 and 21 years old; 213 patients were between 21 and 65 years old and 98 patients were older than 65 years and the ages of 3 male patients were not specified. A racial distribution is not available. In this database, adverse drug reactions that required medical intervention were not reported.
In a small, 42 patient subset of the 374 patients studied, 4 patients had transient hypotension, 6 had hypo- or hyperglycemia and 3 had transient increases in alkaline phosphatase.
-
DOSAGE AND ADMINISTRATION
[ 18F]FDG uptake may be changed by fasting or by blood sugar changes associated with diabetic mellitus. Blood glucose levels should be stabilized in non-diabetic patients by fasting before [ 18F]FDG injection. Diabetic patients may need stabilization of blood glucose on the day preceding and on the day of the [ 18F]FDG scan.
The recommended dose of [ 18F]FDG for an adult (70 kg) is within the range 185-370 MBq (5-10 mCi), intravenous injection. In children doses as low as 2.6 mCi have been given. Optimal dose reductions for children have not been confirmed.
The optimum rate of administration and upper safe dose for [ 18F]FDG have not been established. The time interval between doses of [ 18F]FDG should be long enough to allow substantial decay (physical and biological) of previous administrations.
It is recommended that PET imaging be initiated within 40 minutes of [ 18F]FDG injection.
The final dose for the patient should be calculated using proper decay factors from the time of the EOS, and measured by a suitable radioactivity calibration system before administration. See decay factors in Table 3.
[ 18F]FDG, like other parenteral drug products, should be inspected visually for particulate matter and discoloration before administration, whenever solution and container permit. Preparations containing particulate matter or discoloration should not be administered. They should be disposed of in a safe manner, in compliance with applicable regulations.
[ 18F]FDG should be stored upright in a lead shielded environment at controlled room temperature.
Aseptic techniques and effective shielding should be employed in withdrawing doses for administration to patients. Waterproof gloves and effective shielding should be worn when handling the product.
- OVERDOSE
-
RADIATION DOSIMETRY
The estimated absorbed radiation doses to an average human adult (70 kg) from intravenous injection of 185 MBq (5 mCi) and 370 MBq (10 mCi) of [ 18F]FDG are shown in Table 4. These estimates were calculated based on human 1 data and using the data published by the International Commission on Radiological Protection 2 for [ 18F]FDG.
Table 4. Estimated Absorbed Radiation Doses after intravenous administration of 2-deoxy-2-[ 18F]fluoro-D-glucose, [ 18F]FDG to a 70 kg patient. Organ mGy/185MBq Rads/5mCi mGy/370 MBq rads/10 mCi Bladder Wall 31.45 3.15 62.90 6.29 1Bladder* 11.00 1.10 22.00 2.20 1Bladder** 22.00 2.20 44.00 4.40 Heart 12.03 1.20 24.05 2.41 Brain 4.81 0.48 9.62 0.96 Kidneys 3.88 0.39 7.77 0.78 Uterus 3.70 0.37 7.40 0.74 Ovaries 2.78 0.28 5.55 0.56 Testes 2.78 0.28 5.55 0.56 Adrenals 2.59 0.26 5.18 0.52 Sm Intestine 2.40 0.24 4.81 0.48 ULI wall 2.40 0.24 4.81 0.48 LLI wall 2.96 0.30 5.92 0.59 Stomach wall 2.22 0.22 4.44 0.44 Liver 2.22 0.22 4.44 0.44 Pancreas 2.22 0.22 4.44 0.44 Spleen 2.22 0.22 4.44 0.44 Breast 2.04 0.20 4.07 0.41 Lungs 2.04 0.20 4.07 0.41 Red marrow 2.04 0.20 4.07 0.41 Other Tissue 2.04 0.20 4.07 0.41 Bone Surfaces 1.85 0.18 3.70 0.37 Thyroid 1.70 0.18 3.59 0.36 *With void 1 hour after administration **With void 2 hours after administration.
The [18F]FDG Effective dose equivalent (Adult)2 is 0.027 mSv/MBq.
1Jones, S.C. , Alavi, A., Christman, D., Montanez, I., Wolf, A.P., and Reivich, M. (1982). The Radiation Dosimetry of 2-F-18 fluoro-2-deoxy-D-glucose in man. J. Nucl. Med. 23, 613-617.
2ICRP Publication 53, Volume 18, No. 1-4, 1987, page 76.
-
HOW SUPPLIED
NDC 24468-001-10
Fludeoxyglucose F18 Injection, USP is supplied in a multi-dose, septum capped 10 mL glass vial containing between 148 - 1480 MBq/mL (4 — 500 mCi/mL) of no carrier added 2-deoxy-2-[ 18F]fluoro-D-glucose at end of synthesis, in approximately 6 mL.
This radiopharmaceutical is licensed by the North Dakota Department of Health for distribution to persons authorized to receive the licensed material pursuant to the terms and conditions of a specific license issued by the U.S. Nuclear Regulatory Commission or an Agreement State.
Storage
[ 18F]FDG should be stored upright in a lead shielded container at controlled room temperature.
Storage and disposal of [ 18F]FDG should be in accordance with the regulations and a specific license issued by the U.S. Nuclear Regulatory Commission or an Agreement State.
Expiration Date and Time
Fludeoxyglucose F18 Injection, USP should be used within 12 hours from the end of synthesis, which is provided on the container label.
Caution: Federal Law Prohibits Dispensing Without Prescription.
Manufactured by:
University of North Dakota
Cyclotron and Positron Operations
501 N Columbia Rd., Grand Forks, ND 58203
- LABELING
-
INGREDIENTS AND APPEARANCE
FLUDEOXYGLUCOSE F-18
fludeoxyglucose f-18 injection injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:24468-001 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Fludeoxyglucose F-18 (UNII: 0Z5B2CJX4D) (Fludeoxyglucose F-18 - UNII:0Z5B2CJX4D) Fludeoxyglucose F-18 500 mCi in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:24468-001-10 10 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product 09/14/2009 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA203994 09/14/2009 Labeler - University of North Dakota (031637015) Establishment Name Address ID/FEI Business Operations University of North Dakota 031637015 manufacture(24468-001)


