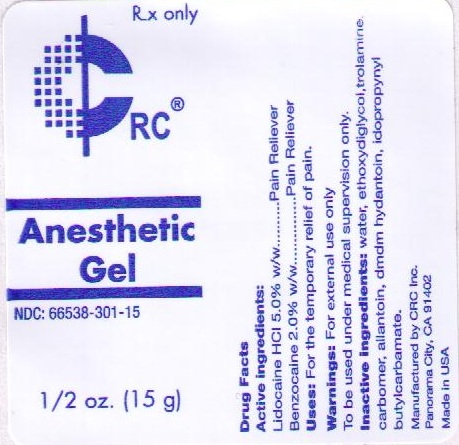
ANESTHETIC- lidocaine hydrochloride benzocaine gel
COSMOCEUTICAL RESEARCH CENTER INC
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
ANESTHETIC GEL
| ANESTHETIC
lidocaine hydrochloride benzocaine gel |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - COSMOCEUTICAL RESEARCH CENTER INC (160019006) |
| Registrant - COSMOCEUTICAL RESEARCH CENTER INC (160019006) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| COSMOCEUTICAL RESEARCH CENTER INC | 160019006 | manufacture(66538-301) | |
Revised: 2/2019
Document Id: c3fadfee-8d8d-44b1-b06e-922c4a73dca0
Set id: 0dd8628f-1fca-4993-adc8-e1e64f2ae39d
Version: 6
Effective Time: 20190207
COSMOCEUTICAL RESEARCH CENTER INC