ALERTNESS AID- caffeine tablet
Chain Drug Consortium
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Premier Value 44-226
Warnings
For occasional use only
Caffeine warning: The recommended dose of this product contains about as much caffeine as a cup of coffee. Limit the use of caffeine-containing medications, foods, or beverages while taking this product because too much caffeine may cause nervousness, irritability, sleeplessness, and, occasionally, rapid heart beat.
Directions
- adults and children 12 years and over: take 1 tablet not more often than every 3 to 4 hours
- children under 12 years: do not use
Other information
- each tablet contains: calcium 35 mg
- TAMPER EVIDENT: DO NOT USE IF OUTER PACKAGE IS OPENED OR BLISTER IS TORN OR BROKEN
- store at 25ºC (77ºF); excursions permitted between 15º-30ºC (59º-86ºF)
- see end flap for expiration date and lot number
Inactive ingredients
corn starch, D&C yellow #10 aluminum lake, dextrates hydrated, dibasic calcium phosphate dihydrate, FD&C yellow #6 aluminum lake, magnesium stearate, microcrystalline cellulose, silicon dioxide
Principal Display Panel
Premier
Value®
*COMPARE TO THE ACTIVE INGREDIENT
IN VIVARIN®
Alertness Aid
Caffeine 200 mg
ALERTNESS AID
Equal to about a cup of coffee
40 Tablets
actual
size
INDEPENDENTLY TESTED
PV
SATISFACTION GUARANTEED
TAMPER EVIDENT: DO NOT USE IF PACKAGE IS OPENED OR IF BLISTER
UNIT IS TORN, BROKEN OR SHOWS ANY SIGNS OF TAMPERING
*This product is not manufactured or distributed by
Meda AB, owner of the registered trademark
Vivarin®. 50844 REV1219A22610
Distributed By:
Pharmacy Value Alliance, LLC
407 East Lancaster Avenue,
Wayne, PA 19087
If for any reason you are not satisfied with
this product, please return it to the store
where purchased for a full refund.
44-226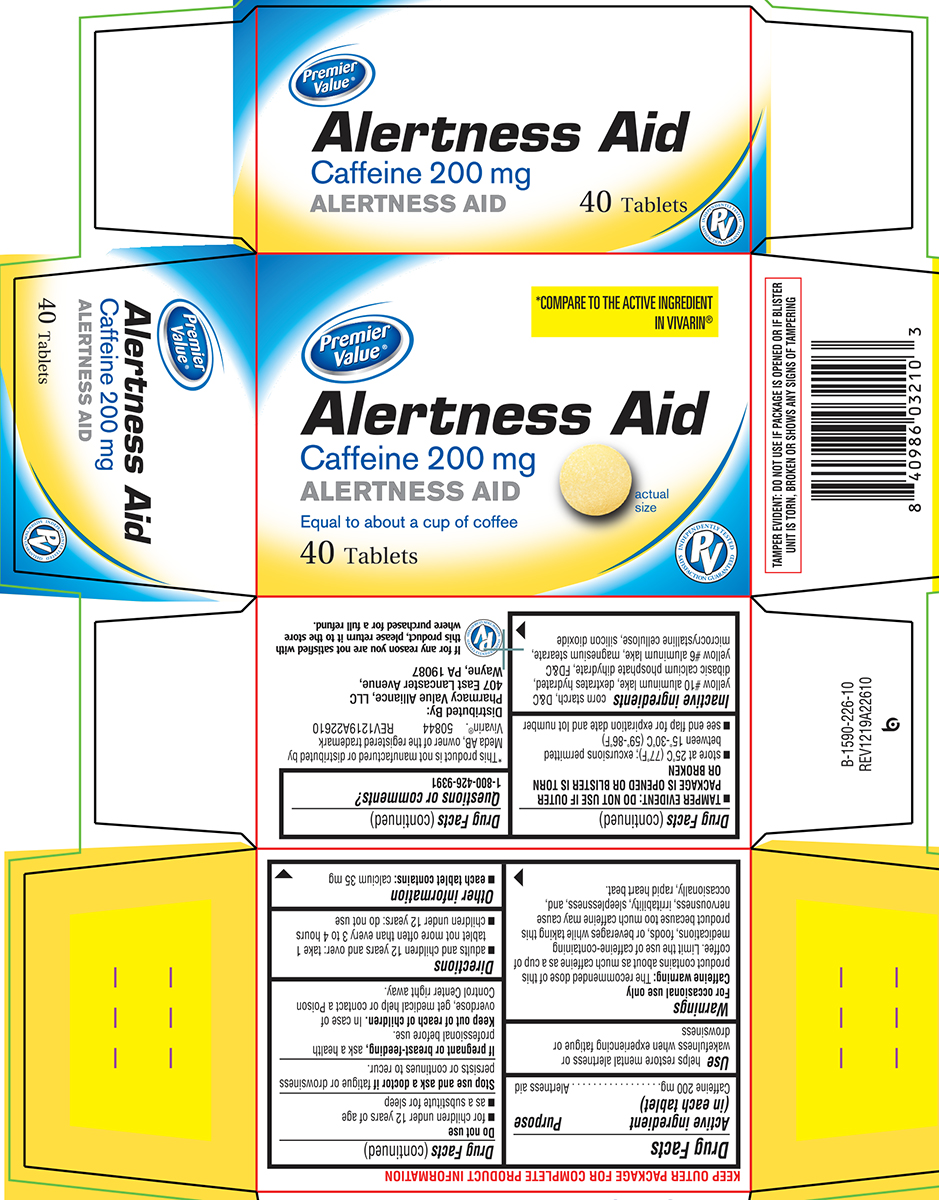
| ALERTNESS AID
caffeine tablet |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Chain Drug Consortium (101668460) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 038154464 | pack(68016-680) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867837 | manufacture(68016-680) , pack(68016-680) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 967626305 | pack(68016-680) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 117025878 | manufacture(68016-680) | |