ADAPALENE AND BENZOYL PEROXIDE- adapalene and benzoyl peroxide gel
TEVA PHARMACEUTICALS USA, INC
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use Adapalene and Benzoyl Peroxide gel safely and effectively. See full prescribing information for Adapalene and Benzoyl Peroxide gel.
Adapalene and Benzoyl Peroxide gel, 0.3%/2.5% is for topical use. Initial U.S. Approval: 2015 INDICATIONS AND USAGEAdapalene and Benzoyl Peroxide gel is a combination of adapalene, a retinoid, and benzoyl peroxide, and is indicated for the topical treatment of acne vulgaris. (1) DOSAGE AND ADMINISTRATIONAdapalene and Benzoyl Peroxide gel is not for oral, ophthalmic, or intravaginal use. (2) Apply a thin layer of Adapalene and Benzoyl Peroxide gel to affected areas of the face and/or trunk once daily after washing. Use a pea-sized amount for each area of the face (e.g., forehead, chin, each cheek). Avoid the eyes, lips, and mucous membranes. (2) DOSAGE FORMS AND STRENGTHSGel, 0.3%/2.5% in 45-g pump (3) CONTRAINDICATIONSNone (4) WARNINGS AND PRECAUTIONS• Ultraviolet Light and Environmental Exposure: Avoid exposure to sunlight and sunlamps. Wear sunscreen when sun exposure cannot be avoided. (5.1) ADVERSE REACTIONSMost commonly reported adverse reactions (≥1%) in patients treated with Adapalene and Benzoyl Peroxide gel were skin irritation, eczema, atopic dermatitis, and skin burning sensation. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Teva Pharmaceuticals USA, Inc. at 1-888-838-2872 or FDA at
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling. Revised: 12/2021 |
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
Adapalene and Benzoyl Peroxide gel is indicated for the topical treatment of acne vulgaris.
2 DOSAGE AND ADMINISTRATION
For topical use only. Adapalene and Benzoyl Peroxide gel is not for oral, ophthalmic, or intravaginal use.
Apply a thin layer of Adapalene and Benzoyl Peroxide gel to affected areas of the face and/or trunk once daily after washing. Use a pea-sized amount for each area of the face (e.g., forehead, chin, each cheek). Avoid the eyes, lips and mucous membranes.
3 DOSAGE FORMS AND STRENGTHS
Each gram of Adapalene and Benzoyl Peroxide gel contains 3 mg (0.3%) adapalene and 25 mg (2.5%) benzoyl peroxide in a white to very pale yellow, opaque gel. Adapalene and Benzoyl Peroxide is available in pump containing 45 g.
5 WARNINGS AND PRECAUTIONS
5.1 Ultraviolet Light and Environmental Exposure
Exposure to sunlight, including sunlamps, should be minimized during the use of Adapalene and Benzoyl Peroxide gel. Patients with high levels of sun exposure and those with inherent sensitivity to sun should exercise particular caution. Use of sunscreen products and protective apparel (e.g., hat) are recommended when exposure cannot be avoided. Weather extremes, such as wind or cold, may be irritating to patients under treatment with Adapalene and Benzoyl Peroxide gel.
5.2 Local Cutaneous Reactions
Erythema, scaling, dryness, and stinging/burning may be experienced with use of Adapalene and Benzoyl Peroxide gel. These are most likely to occur during the first four weeks of treatment, are mostly mild to moderate in intensity, and usually lessen with continued use of the medication. Irritant and allergic contact dermatitis may occur. Depending upon the severity of these adverse reactions, patients should be instructed to use a moisturizer, reduce the frequency of the application of Adapalene and Benzoyl Peroxide gel, or discontinue use. The product should not be applied to cuts, abrasions, eczematous or sunburned skin. As with other retinoids, use of “waxing” as a depilatory method should be avoided on skin treated with Adapalene and Benzoyl Peroxide gel.
Avoid concomitant use of other potentially irritating topical products (medicated or abrasive soaps and cleansers, soaps and cosmetics that have strong skin-drying effect and products with high concentrations of alcohol, astringents, spices, or limes).
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice. During the Phase 3 clinical trial, 217 subjects were exposed to Adapalene and Benzoyl Peroxide gel. A total of 197 subjects with acne vulgaris, 12 years and older, were treated once daily for 12 weeks. Adverse reactions reported within 12 weeks of treatment in at least 1% of subjects treated with Adapalene and Benzoyl Peroxide gel and for which the rate with Adapalene and Benzoyl Peroxide gel exceeded the rate for the vehicle gel are presented in Table 1:
| Adapalene and Benzoyl Peroxide Gel 0.3%/2.5% (N=217) | Adapalene and Benzoyl Peroxide Gel, 0.1%/2.5% (N=217) | Vehicle Gel (N=69) |
|
| Skin irritation | 4% | <1% | 0% |
| Eczema | 1% | 0% | 0% |
| Dermatitis atopic | 1% | 0% | 0% |
| Skin burning sensation | 1% | 0% | 0% |
Local tolerability evaluations presented in Table 2, were conducted at each study visit in the clinical trial by assessment of erythema, scaling, dryness, and stinging/burning, which peaked at Week 1 of therapy and decreased thereafter.
| Maximum Severity During Treatment | End of Treatment Severity (Final Score) |
|||
| Moderate | Severe | Moderate | Severe | |
| Adapalene and Benzoyl Peroxide Gel 0.3%/2.5% (N=213) | ||||
| Erythema | 20% | 1% | 4% | <1% |
| Scaling | 17% | 1% | 1% | <1% |
| Dryness | 15% | 2% | 3% | <1% |
| Stinging/Burning | 19% | 6% | 1% | 1% |
| Adapalene and Benzoyl Peroxide Gel, 0.1%/2.5% (N=212) | ||||
| Erythema | 15% | 1% | 2% | <1% |
| Scaling | 12% | <1% | 2% | 0% |
| Dryness | 13% | 1% | 2% | 0% |
| Stinging/Burning | 14% | 9% | 3% | 0% |
| Vehicle Gel (N=68) | ||||
| Erythema | 6% | 1% | 1% | 0% |
| Scaling | 6% | 0% | 1% | 0% |
| Dryness | 4% | 1% | 1% | 0% |
| Stinging/Burning | 3% | 1% | 0% | 0% |
6.2 Post-Marketing Experience
There is no post-marketing experience with Adapalene and Benzoyl Peroxide gel.
The following adverse reactions have been identified during post-approval use of Adapalene and Benzoyl Peroxide gel, a similar drug containing 0.1% adapalene and 2.5% benzoyl peroxide as the active ingredients: eyelid edema, sunburn, blister, pain of skin, pruritus, swelling face, conjunctivitis, skin discoloration, rash, eczema, throat tightness and allergic contact dermatitis. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
7 DRUG INTERACTIONS
No formal drug-drug interaction studies were conducted with Adapalene and Benzoyl Peroxide gel.
Concomitant topical acne therapy should be used with caution because a possible cumulative irritancy effect may occur, especially with the use of peeling, desquamating, or abrasive agents.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C. There are no well-controlled trials in pregnant women treated with Adapalene and Benzoyl Peroxide gel. Animal reproduction studies have not been conducted with the combination gel. Furthermore, such studies are not always predictive of human response; therefore, Adapalene and Benzoyl Peroxide gel should be used during pregnancy only if the potential benefit justifies the risk to the fetus.
No teratogenic effects were observed in rats treated with oral doses of 0.15 to 5.0 mg adapalene/kg/day, up to 8 times (mg/m2/day) the maximum recommended human dose (MRHD) of 2 grams of Adapalene and Benzoyl Peroxide gel. However, teratogenic changes were observed in rats and rabbits when treated with oral doses of ≥ 25 mg adapalene/kg/day representing 41 and 81 times MRHD, respectively. Findings included cleft palate, microphthalmia, encephalocele, and skeletal abnormalities in rats; and umbilical hernia, exophthalmos, and kidney and skeletal abnormalities in rabbits.
Dermal teratology studies conducted in rats and rabbits at doses of 0.6-6.0 mg adapalene/kg/day (9.7-19.5 times MRHD) exhibited no fetotoxicity and only minimal increases in supernumerary ribs in both species and delayed ossification in rabbits.
8.3 Nursing Mothers
It is not known whether adapalene or benzoyl peroxide is excreted in human milk following use of Adapalene and Benzoyl Peroxide gel. Because many drugs are excreted in human milk, caution should be exercised when Adapalene and Benzoyl Peroxide gel is administered to a nursing woman.
11 DESCRIPTION
Adapalene and Benzoyl Peroxide (adapalene and benzoyl peroxide) gel, 0.3%/2.5% is a white to very pale yellow, opaque gel for topical use containing adapalene 0.3% and benzoyl peroxide 2.5%. Adapalene, a synthetic retinoid, is a naphthoic acid derivative with retinoid-like properties. The chemical name for adapalene is (6-[3-(1-adamantyl)-4-methoxyphenyl]-2-naphthoic acid). It has the following structural formula:
Adapalene:
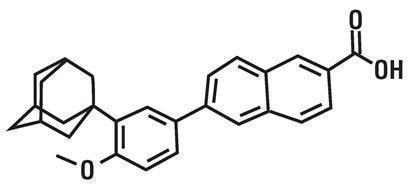
Molecular formula: C28H28O3 Molecular weight: 412.5
Benzoyl Peroxide is a highly lipophilic oxidizing agent that localizes in both bacterial and keratinocyte cell membranes. The chemical name for benzoyl peroxide is dibenzoyl peroxide. It has the following structural formula:
Benzoyl Peroxide:
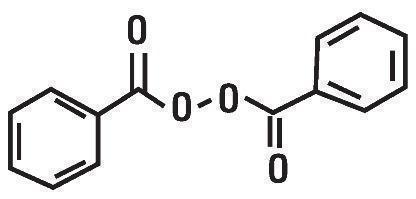
Molecular formula: C14H10O4 Molecular weight: 242.23
Adapalene and Benzoyl Peroxide gel contains the following inactive ingredients: acrylamide/sodium acryloyldimethyltaurate copolymer, docusate sodium, edetate disodium, glycerin, isohexadecane, poloxamer 124, polysorbate 80, propylene glycol, purified water, and sorbitan oleate.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Adapalene
Adapalene binds to specific retinoic acid nuclear receptors but does not bind to cytosolic receptor protein. Biochemical and pharmacological profile studies have demonstrated that adapalene is a modulator of cellular differentiation, keratinization and inflammatory processes. However, the significance of these findings with regard to the mechanism of action of adapalene for the treatment of acne is unknown.
Benzoyl peroxide
Benzoyl peroxide is an oxidizing agent with bactericidal and keratolytic effects.
12.3 Pharmacokinetics
A pharmacokinetic study was conducted in 26 adult and adolescent subjects (12 to 33 years of age) with severe acne vulgaris who were treated with once-daily applications during a 4-week period with, on average, 2.3 grams/day (range 1.6-3.1 grams/day) of Adapalene and Benzoyl Peroxide gel applied as a thin layer to the face, shoulders, upper chest, and upper back.
After a 4-week treatment, 16 subjects (62%) had quantifiable adapalene plasma concentrations above the limit of quantification of 0.1 ng/mL, with a mean Cmax of 0.16 ± 0.08 ng/mL and a mean AUC0-24hr of 2.49 ± 1.21 ng.h/mL. The most exposed subject had adapalene Cmax and AUC0-24hr of 0.35 ng/mL and 6.41 ng.h/mL, respectively. Excretion of adapalene appears to be primarily by the biliary route.
Benzoyl peroxide is absorbed by the skin where it is converted to benzoic acid and eliminated in the urine.
13. NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No carcinogenicity, photocarcinogenicity, genotoxicity, or fertility studies were conducted with Adapalene and Benzoyl Peroxide gel.
Carcinogenicity studies with adapalene have been conducted in mice at topical doses of 0.4, 1.3, and 4.0 mg/kg/day (1.2, 3.9, 12 mg/m2/day), and in rats at oral doses of 0.15, 0.5, and 1.5 mg/kg/day (0.9, 3.0, and 9.0 mg/m2/day). In terms of body surface area, the highest dose levels are 3.2 (mice) and 2.4 (rats) the MRHD of 2 grams of Adapalene and Benzoyl Peroxide gel. In the rat study, an increased incidence of benign and malignant pheochromocytomas reported in the adrenal medulla of male rats was observed.
No significant increase in tumor formation was observed in rodents topically treated with 15-25% benzoyl peroxide carbopol gel (6-10 times the concentration of benzoyl peroxide in Adapalene and Benzoyl Peroxide gel) for two years. Rats received maximum daily applications of 138 (males) and 205 (females) mg benzoyl peroxide/kg. In terms of body surface area, these levels are 27-40 times the MRHD. Similar results were obtained in mice topically treated with 25% benzoyl peroxide carbopol gel for 56 weeks followed by intermittent treatment with 15% benzoyl peroxide carbopol gel for rest of the 2 year study period, and in mice topically treated with 5% benzoyl peroxide carbopol gel for two years.
The role of benzoyl peroxide as a tumor promoter has been well established in several animal species. The significance of this finding in humans is unknown.
In a photocarcinogenicity study conducted with 5% benzoyl peroxide carbopol gel, no increase in UV-induced tumor formation was observed in hairless mice topically treated for 40 weeks.
No photocarcinogenicity studies were conducted with adapalene. However, animal studies have shown an increased tumorigenic risk with the use of pharmacologically similar drugs (e.g., retinoids) when exposed to UV irradiation in the laboratory or sunlight. Although the significance of these findings to humans is not clear, patients should be advised to avoid or minimize exposure to either sunlight or artificial irradiation sources.
Adapalene did not exhibit mutagenic or genotoxic effects in vitro (Ames test, Chinese hamster ovary cell assay, or mouse lymphoma TK assay) or in vivo (mouse micronucleus test).
Bacterial mutagenicity assays (Ames test) with benzoyl peroxide has provided mixed results; mutagenic potential was observed in a few but not in a majority of investigations. It has been shown to produce single-strand DNA breaks in human bronchial epithelial and mouse epidermal cells, caused DNA-protein cross-links in the human cells, and also induced a dose-dependent increase in sister chromatid exchanges in Chinese hamster ovary cells.
In rat oral studies, 20 mg adapalene/kg/day did not affect the reproductive performance and fertility of F0 males and females, or the growth, development and reproductive function of F1 offspring.
No fertility studies were conducted with benzoyl peroxide.
14 CLINICAL STUDIES
The safety and efficacy of Adapalene and Benzoyl Peroxide gel applied once daily for 12 weeks for the treatment of acne vulgaris were assessed in a multicenter, randomized, double-blind, vehicle-controlled study, comparing Adapalene and Benzoyl Peroxide gel to vehicle gel in subjects with acne vulgaris. The study also evaluated Adapalene and Benzoyl Peroxide gel, 0.1%/2.5%, a lower strength product than Adapalene and Benzoyl Peroxide gel, 0.3%/2.5%. In this study, 217 subjects were treated with Adapalene and Benzoyl Peroxide gel 0.3%/2.5%, 217 subjects with Adapalene and Benzoyl Peroxide, gel, 0.1%/2.5% and 69 subjects with the vehicle gel.
Treatment response was defined as the percent of subjects who were rated “clear” or “almost clear” at Week 12 with at least a two-grade improvement based on the Investigator’s Global Assessment (IGA), and mean absolute change from baseline at Week 12 in both inflammatory and non-inflammatory lesion counts. An IGA score of ‘Clear’ corresponded to clear skin with no inflammatory or non-inflammatory lesions. An IGA score of “almost clear” corresponded to a few scattered comedones and a few small papules.
At baseline, 50% of subjects were graded as “moderate” (IGA Grade 3) and 50% were graded as “severe” (IGA Grade 4) on the IGA scale. Subjects had an average of 98 (range 51-226) total lesions of which the mean number of inflammatory lesions was 38 (range: 20-99) and the mean number of non-inflammatory lesions was 60 (range 30-149). Subjects ranged in age from 12 to 57 years, with 273 (54%) of subjects 12 to 17 years of age. Approximately equal number of males (48%) and females (52%) were enrolled.
The IGA success rate, mean reduction, and percent reduction in acne lesion counts from baseline after 12 weeks of treatment are presented in the following table.
| Adapalene and Benzoyl Peroxide Gel 0.3%/2.5% (N=217) | Adapalene and Benzoyl Peroxide Gel, 0.1%/2.5% (N=217) * | Vehicle Gel (N=69) |
|
| IGA: two-grade improvement and "clear" or "almost clear" | 33.7% | 27.3% | 11.0% |
| Inflammatory lesions: mean absolute (percent) reduction | 27.8 (68.7%) | 26.5 (69.3%) | 13.2 (39.2%) |
| Non-inflammatory lesions: mean absolute (percent) reduction | 40.5 (68.3%) | 40.0 (68.0%) | 19.7 (37.4%) |
* This study was not designed or powered to compare the efficacy of Adapalene and Benzoyl Peroxide gel to the lower strength Adapalene and Benzoyl Peroxide gel, 0.1%/2.5%, nor to compare the lower strength adapalene and Benzoyl Peroxide Gel, 0.1%/2.5% to the vehicle control.
In subjects graded as “severe” (IGA Grade 4), efficacy was observed in the Adapalene and Benzoyl Peroxide group.
16 HOW SUPPLIED/STORAGE AND HANDLING
Adapalene and Benzoyl Peroxide gel 0.3%/2.5% is white to very pale yellow in color and opaque in appearance, and is supplied as follows:
45 gram pump NDC 0093-6303-45
Storage and handling
• Store at controlled room temperature 20–25°C (68–77°F) with excursions permitted to 15°–30°C (59°–86°F) [see USP controlled room temperature].
• Protect from light.
• Keep out of reach of children.
• Keep away from heat.
17 PATIENT COUNSELING INFORMATION
[See FDA Approved Patient Labeling (Patient Information)]
Information for Patients
• Advise patients to cleanse the area to be treated with a mild or soapless cleanser; pat dry. Apply Adapalene and Benzoyl Peroxide gel as a thin layer, avoiding the eyes, lips and mucous membranes.
• Advise patients not to use more than the recommended amount and not to apply more than once daily as this will not produce faster results, but may increase irritation.
• Adapalene and Benzoyl Peroxide gel may cause irritation such as erythema, scaling, dryness, stinging or burning.
• Advise patients to minimize exposure to sunlight, including sunlamps.
• Recommend the use of sunscreen products and protective apparel (e.g., hat) when exposure cannot be avoided.
• Adapalene and Benzoyl Peroxide gel may bleach hair and colored fabric.
Manufactured for:
Teva Pharmaceuticals USA, Inc.
Parsippany, NJ 07054
Made in Canada
P55960-0
Rev. 04/2021
Patient Information
Adapalene and Benzoyl Peroxide gel 0.3%/2.5%
Important Information: Adapalene and Benzoyl Peroxide gel is for use on the skin only (topical). Do not useAdapalene and Benzoyl Peroxide gel in or on your mouth, eyes, or vagina.
What is Adapalene and Benzoyl Peroxide gel?
Adapalene and Benzoyl Peroxide gel is a prescription medicine used on the skin (topical) to treat acne vulgaris. It is not known whether Adapalene and Benzoyl Peroxide gel is safe and effective in children under 12 years of age.
Before using Adapalene and Benzoyl Peroxide gel, tell your doctor about all of your medical conditions, including if you:
• have other skin problems, including cuts or sunburn
• are pregnant or plan to become pregnant. It is not known if Adapalene and Benzoyl Peroxide gel can harm your unborn baby. Talk to your doctor if you are pregnant or plan to become pregnant.
• are breastfeeding or plan to breastfeed. It is not known if Adapalene and Benzoyl Peroxide gel passes into your breast milk and if it can harm your baby. Talk to your doctor about the best way to feed your baby if you use Adapalene and Benzoyl Peroxide gel.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Using other topical acne products may increase the irritation of your skin when used with Adapalene and Benzoyl Peroxide gel.
How should I use Adapalene and Benzoyl Peroxide gel?
• Use Adapalene and Benzoyl Peroxide gel exactly as your doctor tells you to use it.
• Apply Adapalene and Benzoyl Peroxide gel 1 time a day.
• Do not use more Adapalene and Benzoyl Peroxide gel than you need to cover the treatment area. Using too much Adapalene and Benzoyl Peroxide gel or using it more than 1 time a day may increase your chance of skin irritation.
Applying Adapalene and Benzoyl Peroxide gel:
• Wash the area where the gel will be applied with a mild or soapless cleanser and pat dry.
• Adapalene and Benzoyl Peroxide gel comes in a pump. Depress the pump to dispense a small amount (about the size of a pea) of Adapalene and Benzoyl Peroxide gel and spread a thin layer over the affected area.
• Wash your hands after applying the gel.
What should I avoid while using Adapalene and Benzoyl Peroxide gel?
• Avoid spending time in sunlight or artificial sunlight, such as tanning beds or sunlamps. Adapalene and Benzoyl Peroxide gel can make your skin sensitive to sun and the light from tanning beds and sunlamps. Use sunscreen and wear a hat and clothes that cover the areas treated with Adapalene and Benzoyl Peroxide gel if you have to be in sunlight.
• Cold weather and wind may irritate skin treated with Adapalene and Benzoyl Peroxide gel.
• Avoid applying Adapalene and Benzoyl Peroxide gel to cuts, abrasions, and sunburned skin.
• Avoid skin products that may dry or irritate your skin such as medicated or harsh soaps, astringents, cosmetics that make your skin dry, and products containing high levels of alcohol, spices, or limes.
• Avoid the use of “waxing” as a hair removal method on skin treated with Adapalene and Benzoyl Peroxide gel.
• Adapalene and Benzoyl Peroxide gel may bleach your clothes or hair. Allow Adapalene and Benzoyl Peroxide gel to dry completely before dressing to prevent bleaching of your clothes.
What are the possible side effects of Adapalene and Benzoyl Peroxide gel?
Adapalene and Benzoyl Peroxide gel may cause serious side effects including:
Local skin reactions. Local skin reactions are most likely to happen during the first 4 weeks of treatment and usually lessen with continued use of Adapalene and Benzoyl Peroxide gel. Signs and symptoms of local skin reactions include redness, scaling, dryness, stinging, or burning.
Tell your doctor right away if these side effects continue for longer than 4 weeks or get worse, you may have to stop using Adapalene and Benzoyl Peroxide gel.
These are not all the possible side effects of Adapalene and Benzoyl Peroxide gel. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. You may also report side effects to Teva Pharmaceuticals USA, Inc. at 1-888-838-2872
How should I store Adapalene and Benzoyl Peroxide gel?
• Store Adapalene and Benzoyl Peroxide gel at room temperature between 68°F to 77°F (20°C to 25°C).
• Keep Adapalene and Benzoyl Peroxide gel out of light and away from heat.
Keep Adapalene and Benzoyl Peroxide gel and all medicines out of the reach of children.
General information about the safe and effective use of Adapalene and Benzoyl Peroxide gel
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Adapalene and Benzoyl Peroxide gel for a condition for which it was not prescribed. Do not give Adapalene and Benzoyl Peroxide gel to other people, even if they have the same symptoms you have. It may harm them. You can ask your doctor or pharmacist for information about Adapalene and Benzoyl Peroxide gel that is written for health professionals.
What are the ingredients in Adapalene and Benzoyl Peroxide gel?
Active ingredient: adapalene and benzoyl peroxide
Inactive ingredients: acrylamide/sodium acryloydimethyltaurate copolymer, docusate sodium, edetate disodium, glycerin, isohexadecane, polaxamer 124, polysorbate 80, propylene glycol, purified water and sorbitan oleate
Manufactured for: Teva Pharmaceuticals, Inc., Parsippany, NJ 07054
This Patient Information has been approved by the U.S. Food and Drug Administration.
Issued: 07/2015
LABEL - 45g Carton
NDC 0093-6303-45
Adapalene and Benzoyl Peroxide Gel
0.3%/2.5%
PUMP
FOR TOPICAL USE ONLY
Rx only
NET WT. 45 g
TEVA
For topical use only.
Not for ophthalmic, oral or intravaginal use.
Usual dosage: Apply a thin layer once a day to affected areas. See package insert for complete prescribing information.
Each gram contains: Active: adapalene 0.3% and benzoyl peroxide 2.5% in a gel. Inactive: acrylamide/sodium acryloyldimethyltaurate copolymer, docusate sodium, edetate disodium, glycerin, isohexadecane, poloxamer 124, polysorbate 80, propylene glycol, purified water, and sorbitan oleate.
Storage: Store at controlled room temperature 68° to 77°F (20° to 25°C) with excursions permitted between 59° and 86°F (15° and 30°C).
See carton closure for lot number and expiration date.
Manufactured for:
Teva Pharmaceuticals USA, Inc.
Parsippany, NJ 07054
Made in Canada.
All trademarks are the property of their respective owners
Rev. 04/2021
P55959-0
| ADAPALENE AND BENZOYL PEROXIDE
adapalene and benzoyl peroxide gel |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - TEVA PHARMACEUTICALS USA, INC (001627975) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| G Production Inc. | 251676961 | manufacture(0093-6303) | |